Table 5.
Amidine Synthesis via Aza-Claisen Rearrangement.
| entry | ynamides | aminesa | amidine products | yield [%]b |
|---|---|---|---|---|
| 1 |
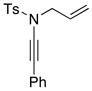 8b 8b
|
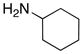
|
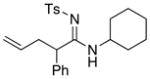 56 56
|
94 |
| 2 |
 8d 8d
|
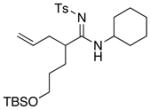 57 57
|
58 | |
| 3 |
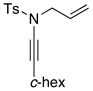 8k 8k
|
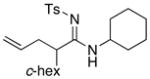 58 58
|
77 | |
| 4 |
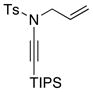 8a 8a
|
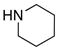
|
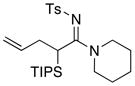 59 59
|
≥95 |
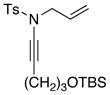
| 5 |
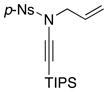 8f 8f
|
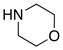
|
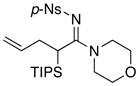 60 60
|
93 |
All reactions utilized 3.0 equiv of the amine and were run in toluene [concn = 0.05 M] at 110 °C over 24 h except it was 48 h for entry 4.
Isolated yields.
