Genetic differences in chemosensory perception of alcohol (taste, smell, and chemical irritation) influence significantly the alcohol preference in both animals and humans [1]. To find out the phenotypic markers of alcohol addiction, of special interest is the positive association between the preference of sweet solutions and an increased alcohol intake, which are characteristic of both rodents [2–4] and humans [5]. The genetic nature of sweet preference has been first proved in [6], where the Sac locus responsible for a high saccharin consumption was identified in mice. The gene Tas1r3 [7] localized to the fourth mouse and first human chromosomes was later established to correspond to the Sac locus and encode T1R3 protein in receptor cells of mammalian taste epithelium [8]. The heterodimeric protein complex T1R2/T1R3 [7, 9, 10] plays the major role in the sweet taste perception [11]. Inbred mouse strains carrying the Sacb taster allele, e.g. the C57BL/6J strain [6, 7], which prefer sweet solutions and ethanol to water, are assumed to be prone to alcohol consumption because of reacting to the sweet component of its taste [3]. Indeed, the strains with less sensitive (non-taster) allele (Sacd for strain 129P3/J) [12] and especially Tas1r3 knockouts [7, 9, 13], which were derived from the strains with the taster allele Sac, exhibited much less preference for sugar and alcohol solutions in long-term preference tests. However, these testing techniques, although most commonly used, do not permit differentiating the role of taste from the postabsorptive effects (intoxicating and metabolic) in the reaction of alcohol preference. Further study is also required to confirm the perception of the ethanol sweet component by mice, as well as the fact that T1R3 protein is responsible for the perception. In this study, we used the brief-access taste test minimizing the time of contact with the test substance and reducing the volume of its consumption. As a result, the contribution of postabsorptive effects is reduced. Here, we analyzed the response of C57BL/6ByJ, 129P3/J, and Tas1r3-knockout mice to alcohol and two prototypical taste components, sweet and bitter. The presence of the non-taster allele of the Sacd locus in 129P3/J mice or complete absence of the Sac locus (Tas1r3−/− mice) was found to predetermine rejection of ethanol. Note that Sac affected neither perception of the bitter component nor the gustatory detection of ethanol.
This study, approved by the Bioethics Committee of Pavlov Institute of Physiology, was performed on four- to six-month-old mice weighing 20–30 g (both males and females). Two inbred strains, 129P3/J (129; n = 31) and C57BL/6ByJ (B6; n = 37) were purchased from the Jackson Laboratory, Bar Harbor, United States. The gene-knockout Tas1r3−/− mice (n = 14) derived from C57BL/6ByJ [13] were kindly provided by Dr. R.F. Margolskee (Mount Sinai School of Medicine, NY, United States). During the brief-access test, controlled presentation and measurement of the consumption of small volumes of taste substances were performed using a Davis MS-160 licometer (DiLog Instruments, Tallahassee, FL, United States), which recorded the number of tongue licking movements and the inter-lick intervals (ILI), as well as the latency to start licking. Before testing, the animals did not receive water for 22–23 h (total water deprivation for testing ethanol and quinine and partial deprivation for sucrose testing) [14]. Aqueous solutions of ethanol (1.25–20%), sucrose (1–32%), and quinine hydrochloride (0.01–1 mM) were used in separate experimental series consisting of 24 5-s presentations of five to six concentrations of a substance alternating with water (5 s is the time of access to a sipper tube after beginning of licking). The solutions were presented in the order of increasing concentrations three times per series. Association of ethanol taste with its prototypical taste components was evaluated using generalization of the conditioned taste aversion (CTA) induced to 10% ethanol to quinine and sucrose solutions (LiCl, i.p., 0.23 g/kg B.W., served as an unconditioned stimulus) [4]. Statistical differences were determined for the calculated index of standardized lick ratio (SLR) of a taste agent [14]. The SLR was calculated for each concentration as the ratio of the average number of lickings per trial to the expected maximum rate of water licking. The latter value was determined by dividing the trial duration (5 s) to the mean ILI [15] for water at the stage of training. The SLR coefficient close to 1.0 means the maximum level of animal preference for the given concentration of the substance. The SLR approaching 0 indicates an aversive taste response. The results were analysed using the Statistica 7.0 software (StatSoft, Tulsa, United States) for each taste agent and multilevel three-way ANOVA, where the factors were the genotype (mouse strain), exposure (CTA), and concentration. A post-hoc analysis was performed using Fisher’s test. The data are presented as mean values ± error of the mean.
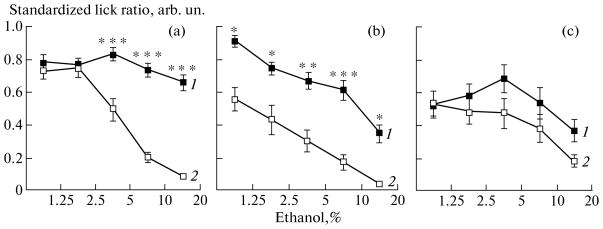
As determined using three-way ANOVA (the genotype effect, F (2, 113) = 6.08, p < 0.001), the Sac genotype had a strong influence on alcohol preference in the brief-access test (figure). Interstrain differences were also dependent on the concentration of substances (F(8, 452) = 4.17, p < 0.001) and on CTA (F(8, 452) = 4.70, p < 0.001). Intact mice carrying the taster allele Sacb (strain B6) exhibited a similar level of consumption of all ethanol concentrations, which did not differ from the consumption of the neutral stimulus (water). In mice carrying the non-taster allele (129), ethanol rejection with increasing concentrations confirmed the role of genotype in general; however, it was not a sufficient proof of the influence of the Sac locus.
The data on the lowest ethanol consumption in gene-knockout Tas1r3−/− mice, which were obtained for the first time (Fig. 1c), serve as the necessary proof of direct dependence of ethanol chemosensory preference on the Sac locus. The major effect of the Sac genotype is likely to be a hedonic response to ethanol, rather than its chemosensory detection. The genotypes studied had similar sensitivities to ethanol, which is confirmed by similar concentration-dependent ethanol rejections after CTA development (Fig. 1). The methodology of the brief-access test implies that changes in the latency of the animal’s approaching the sipper tube characterizes the effect of certain factors, such as olfaction and learning [14]. The concentration dependence (F(8,264) = 3.34, p < 0.001) confirms that changes in the latency of approaching the sipper tube depended on the effect of ethanol vapor on olfaction. The maximum latency, which is likely to reflect the triggering of unconditioned avoidance via the olfactory perception, was observed in mice of the strain 129 and in gene-knock-out mice. In the latter, the latency was at least two times longer in the experimental trials with 5 and 20% solutions. The negative response to ethanol odor increased after CTA; a significant increase in the latency was observed in all groups (F(4,264) = 7.40, p < 0.001).
Fig 1.
Consumption of an ethanol solution in a brief-access test by inbred mice of strains (a) C57BL/6ByJ, (b) 129P3/J, and (c) Tas1r3−/− (gene-knockout mice). The effect of conditioned taste aversion (CTA) to 10% ethanol to the ethanol consumption: 1, the response of intact animals; 2, the response after CTA. Statistical comparison was performed using ANOVA and Fisher’s test; ***p < 0.001; **p < 0.01; *p < 0.05.
The hedonic response to the bitter quinine solution was independent of the Sac locus: no differences were found between the intact mice B6 and Tas1r3−/−, which equally avoided quinine beginning from 0.3 mM. Nevertheless, the response to bitter clearly depended on the genotype, because the intact mice of strain 129 avoided quinine beginning from 0.03 mM more actively than B6 and Tas1r3−/− mice. A significant association between the ethanol and quinine tastes was observed in strains B6 and Tas1r3−/−, which generalized CTA from 10% ethanol to quinine (aversion effect, F(4, 488) = 2.55, p < 0.03). No significant changes in the quinine consumption after CTA was found in strain 129; however, an increase in the latency of approaching quinine after CTA (p < 0.05) suggests that the animals of this strain also differentiated the bitter component of the ethanol taste.
As expected, there was significant association between the genotype and a preference for sucrose solutions; in particular, the association with the Sac locus (F(2, 106) = 19.14 at p < 0.001) was concentration-dependent (F(10, 530) = 3.18 at p < 0.001). After CTA, the difference between the genotypes was still observed: intact mice of strain 129 and, especially, Tas1r3−/− mice consumed a lower amount of 1–8% sucrose than B6 mice (p < 0.05 and p < 0.01, respectively). Unlike the previous data on CTA generalization from low sucrose concentration (50 mM) to 10% ethanol in the B6 strain during the long-term free-choice testing [4], in our experiments, no generalization of the learned avoidance from 10% ethanol to sucrose was observed in any genotype. This was probably a result of the difference in the method of testing; in our case, there was no effect of novelty of the stimulus (sucrose) for the mice, which were familiar with all the taste stimuli prior to CTA generation; thus, they readily identified the dominant bitter component of the ethanol taste to respond primarily with CTA to this component. Our comparative study of consumption of alcohol, sucrose, and quinine solutions in the brief-access test by mice of different strains confirmed that interdependence of alcohol and sweet preference is related to the genetically determined features of chemosensory perception of these substances.
Ethanol preference was predominantly determined by the expression of the Tas1r3 allele (locus Sac). The presence of only recessive non-taster alleles of Sacd in the 129P3/J strain and a complete absence of the Sac locus in gene-knockout Tas1r3−/− mice predetermined behavioral rejection of ethanol (probably, because of avoidance of the bitter component of its taste) and a lower preference for sweet solutions. In C57BL/6ByJ mice, the presence of the Sacb taster allele responsible for a high sensitivity to sweet taste probably compensates for the aversive perception of bitter taste and ethanol smell, which promotes the reaction of preference. The Sac locus proved to have no influence on chemosensory detection of ethanol solutions, which is based on recognizing the bitter taste component and odor.
Acknowledgments
This study was supported by the National Institutes of Health (United States), grant no. R03TW007429, R01 AA011028 and R01 DC000882.
References
- 1.Bachmanov AA, Kiefer SW, Molina JC, Tordoff MG, Duffy VB, Bartoshuk LM, Mennella JA. Chemosensory factors influencing alcohol perception, preferences, and consumption. Alcohol Clin Exp Res. 2003 Feb;27(2):220–31. doi: 10.1097/01.ALC.0000051021.99641.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bachmanov AA, Reed DR, Tordoff MG, et al. Behav Genet. 1996;26(6):563–573. doi: 10.1007/BF02361229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blednov YA, Walker D, Martinez M, et al. Genes Brain Behav. 2008;7:1–13. doi: 10.1111/j.1601-183X.2007.00309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blizard DA. Behav Genet. 2007;37:146–159. doi: 10.1007/s10519-006-9121-4. [DOI] [PubMed] [Google Scholar]
- 5.Kampov-Polevoy AB, Tsoi MV, Zvartau EE, Neznanov NG, Khalitov E. Sweet liking and family history of alcoholism in hospitalized alcoholic and non-alcoholic patients. Alcohol Alcohol. 2001 Mar-Apr;36(2):165–70. doi: 10.1093/alcalc/36.2.165. [DOI] [PubMed] [Google Scholar]
- 6.Fuller JL. Single-locus control of saccharin preference in mice. J Hered. 1974 Jan-Feb;65(1):33–6. doi: 10.1093/oxfordjournals.jhered.a108452. No abstract available. [DOI] [PubMed] [Google Scholar]
- 7.Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJ, Zuker CS. Mammalian sweet taste receptors. Cell. 2001 Aug 10;106(3):381–90. doi: 10.1016/s0092-8674(01)00451-2. [DOI] [PubMed] [Google Scholar]
- 8.Bachmanov AA, Li X, Reed DR, et al. Chem Senses. 2001;26:925–933. doi: 10.1093/chemse/26.7.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao GQ, Zhang Y, Hoon MA, et al. Cell. 2003;115:255–266. doi: 10.1016/s0092-8674(03)00844-4. [DOI] [PubMed] [Google Scholar]
- 10.Xu H, Staszewski L, Tang H, et al. Proc Natl Acad Sci USA. 2004;101(39):14258–14263. doi: 10.1073/pnas.0404384101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chandrashekar J, Hoon MA, Ryba NJP, et al. Nature. 2006;444(16):288–294. doi: 10.1038/nature05401. [DOI] [PubMed] [Google Scholar]
- 12.Inoue M, Glendinning JI, Theodorides ML, Harkness S, Li X, Bosak N, Beauchamp GK, Bachmanov AA. Allelic variation of the Tas1r3 taste receptor gene selectively affects taste responses to sweeteners: evidence from 129.B6-Tas1r3 congenic mice. Physiol Genomics. 2007 Dec 19;32(1):82–94. doi: 10.1152/physiolgenomics.00161.2007. Epub 2007 Oct 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Damak S, Rong M, Yasumatsu K, et al. Science. 2003;301:850–853. doi: 10.1126/science.1087155. [DOI] [PubMed] [Google Scholar]
- 14.Glendinning JI, Gresack J, Spector AC. Chem Senses. 2002;27:461–474. doi: 10.1093/chemse/27.5.461. [DOI] [PubMed] [Google Scholar]
- 15.Boughter JD, Jr, Baird JP, Bryant J, et al. Genes Brain Behav. 2007;6:619–627. doi: 10.1111/j.1601-183X.2006.00293.x. [DOI] [PubMed] [Google Scholar]