Abstract
OBJECTIVE
To determine the effect of metformin on the acute metabolic response to submaximal exercise, the effect of exercise on plasma metformin concentrations, and the interaction between metformin and exercise on the subsequent response to a standardized meal.
RESEARCH DESIGN AND METHODS
Ten participants with type 2 diabetes were recruited for this randomized crossover study. Metformin or placebo was given for 28 days, followed by the alternate condition for 28 days. On the last 2 days of each condition, participants were assessed during a nonexercise and a subsequent exercise day. Exercise took place in the morning and involved a total of 35 min performed at three different submaximal intensities.
RESULTS
Metformin increased heart rate and plasma lactate during exercise (both P ≤ 0.01) but lowered respiratory exchange ratio (P = 0.03) without affecting total energy expenditure, which suggests increased fat oxidation. Metformin plasma concentrations were greater at several, but not all, time points on the exercise day compared with the nonexercise day. The glycemic response to a standardized meal was reduced by metformin, but the reduction was attenuated when exercise was added (metformin × exercise interaction, P = 0.05). Glucagon levels were highest in the combined exercise and metformin condition.
CONCLUSIONS
This study reveals several ways by which metformin and exercise therapies can affect each other. By increasing heart rate, metformin could lead to the prescription of lower exercise workloads. Furthermore, under the tested conditions, exercise interfered with the glucose-lowering effect of metformin.
It is estimated that there were over 42 million prescriptions for metformin in the U.S. in 2009 (top 10 for generic drugs) (1). Along with these prescriptions, exercise had likely been recommended to most of these patients since metformin therapy and lifestyle modifications are considered the first step for the management of type 2 diabetes (2).
Despite the vast literature examining the effects of metformin or exercise separately, surprisingly few studies have examined how they affect each other, or if their combination offers additive benefits. There is some evidence suggesting that the benefits of exercise and metformin are not additive. For example, in the Indian Diabetes Prevention Program, reductions in the risk of diabetes were similar in the combined metformin and lifestyle modification group (−28.2%) compared with the metformin (−26.4%) or lifestyle alone (−28.5%) groups (3). In addition, metformin has been recently suggested to attenuate the insulin-sensitizing effect of exercise (4).
Muscle contraction is known to result in the metabolic conditions that lead to activation of AMP-activated protein kinase (AMPK), and there is growing evidence that metformin also increases AMPK activity in liver, muscle, and other tissues (5). Recently, there has been much attention given to AMPK activators as exercise mimetics (6) and metformin has been shown to improve exercise tolerance in nondiabetic women with clinically defined angina (7).
The objectives of the current exploratory study were threefold: 1) to examine the effect of metformin on the acute metabolic and hormonal responses to exercise, 2) to examine the effect of exercise on plasma metformin concentrations, and 3) to examine the interaction between metformin and acute exercise on the subsequent response to a standardized meal.
RESEARCH DESIGN AND METHODS
Participants
Ten volunteers (eight men and two postmenopausal women) with type 2 diabetes were recruited for this study, which was approved by the University Health Research Ethics Board. Participants met the following eligibility criteria: 1) between 30 and 65 years of age; 2) not taking glucose-lowering medication or insulin; 3) no changes in physical activity over the last 3 months and not planning on changing medication, physical activity, or diet over the course of the study; and 4) HbA1c ≤8%, resting blood pressure ≤140/90 mmHg, LDL cholesterol ≤3.5 mmol/L, and total:HDL cholesterol ≤5.0.
Study design
The study used a factorial design and each participant was exposed to 4 conditions: 1) metformin and no exercise, 2) metformin and exercise, 3) placebo and no exercise, and 4) placebo and exercise. The order of the metformin versus placebo conditions was randomized by personnel not involved with the study, and allocation was concealed in sealed envelopes until participants completed the study. Participants, study personnel, and investigators were blinded to the order of the placebo/metformin conditions. Metformin or placebo was given for 28 days, immediately followed by the alternate condition for 28 days. On the last 2 days of each condition (days 27 and 28), participants returned to the Exercise Physiology Laboratory for a nonexercise and exercise session, respectively. The order of these sessions was not randomly determined. Exercise was always performed on day 28 since the acute glucose-lowering effect of exercise may persist for at least 24 h (8).
Study protocol
During the baseline, an exercise stress test with a 12-lead electrocardiogram was performed using a modified Balke-Ware treadmill protocol. Each participant walked at a self-selected speed, determined as comfortable but fast, while the grade was increased by 2% each minute. The test was ended when the participant could no longer continue. This protocol was used to determine the peak oxygen uptake (Vo2peak) and ventilatory threshold using the V-slope method.
After the baseline visit, participants were given either metformin or placebo pills and were asked to maintain their routine physical activity and dietary habits. Each participant consumed 500 mg of metformin with breakfast during the first week of the intervention followed by a 500-mg increase in each of the subsequent weeks until 1,000 mg were consumed with breakfast and supper during week 4 (total: 2,000 mg/day).
On days 27 and 28 of the metformin and placebo conditions, participants arrived in the laboratory at 8:00 a.m. after a 12-h fast. Fasting glucose was measured with a handheld glucose meter (One Touch Ultra; LifeScan, Milpitas, CA), and then participants ate a standardized breakfast (549 kcal; 56% carbohydrate, 30% fat, 14% protein) and took their assigned pills. At 10:00 a.m., an intravenous catheter was inserted into an antecubital vein kept patent with 0.9% sterile saline and the first blood sample was collected at 10:20 a.m. On day 27 of the metformin and placebo conditions, the participants remained at rest for the duration of the testing period. At 10:45 a.m. of day 28 during both conditions, participants performed a series of exercises that were selected to represent different intensities, modes, and energy systems. They began with 20 consecutive maximal leg extensions and flexions on a Cybex II isokinetic dynamometer (Lumex). The isokinetic test was included since resistance exercise is recommended for people with diabetes (9) and since isokinetic testing helps avoid eccentric contractions under load, which might induce muscle damage and impact the subsequent treadmill tests. The angular velocity was set at 180° × s−1 (3.14 rads × s−1), and measurements of peak torque, mean torque, and a fatigue index were calculated. After a 5-min rest period, the first of three aerobic exercise bouts began. Each bout was separated by a 5-min rest period during which blood samples were drawn from the catheter. During the first exercise bout, all participants walked at 3.5 km/h and 0% grade for 15 min. This corresponded to the estimated average walking speed for individuals with type 2 diabetes in free-living conditions (10). The second bout also lasted 15 min and was completed at a speed and grade equivalent to an intensity below each participant’s measured ventilatory threshold. The third bout was completed at an intensity above their ventilatory threshold and lasted 5 min.
Metabolic outcomes such as the volume of oxygen consumed (Vo2) and the volume of carbon dioxide produced (Vco2) during exercise were measured with a TrueMax metabolic measurement system (Parvo Medics, Salt Lake City, UTtah). Heart rate (HR) was measured using a Polar heart rate monitor (Polar Electric, Finland), and rate of perceived exertion was estimated with the Borg Scale.
About 20 min after exercise (at 11:59 a.m.), another blood sample was taken immediately before the standardized meal (556 kcal; 59% carbohydrate, 22% fat, 19% protein). Participants remained in the laboratory, and blood samples were taken every 30 min for 2 h.
Each blood sample was first transferred into a 10-mL EDTA vacutainer tube. Subsequently, 0.25 mL whole blood was transferred into 1.0 mL ice-cold 8% perchloric acid, and 2.0 mL was transferred into a tube with 67 μL aprotinin. Perchloric acid was added for deproteinization as required for the lactate analysis. Aprotinin was added to eliminate proteases known to interfere with the determination of glucagon. Tubes were centrifuged and cooled before being moved to a −20°C freezer until assays were completed. Serum lactate, glucose, and nonesterified fatty acids (NEFAs) were determined enzymatically with spectrophotometric assays. Glucagon and insulin were measured using commercially available radioimmunoassay (RIA) kits (Millipore, St. Charles, MO and Inter Medico, Markham, Ontario, Canada, respectively). All assays were run in duplicate.
Plasma metformin concentrations were assessed in all plasma samples by a high performance liquid chromatographic. The concentration of phosphate solution used in the mobile phase was 20 mmol/L. The assay was validated to a lower limit of quantitation of 7.8 ng/mL metformin based on 0.1 mL of human plasma (11).
Potential confounding variables
Participants completed a 24-h food recall for the day preceeding each testing session. These were analyzed for calorie and macronutrient content using Food Processor SQL (Version 8.3.0; ESHA Research, Salem, OR). Habitual physical activity during each 4-week intervention was assessed using the Godin Leisure Time questionnaire (12). Finally, participants were asked to indicate their perception of which intervention (metformin or placebo) they had just completed on a 150-mm visual analog scales as well as symptoms such as nausea, headache, flatulence, abdominal discomfort, and indigestion.
Statistical analyses
Analyses were conducted using repeated-measures ANOVA with treatment order added as a between-subject factor. To simplify the interpretation, the testing days were broken down into four periods: pre-exercise, exercise, postexercise, and postlunch. The number of within-factors and levels varied among these periods (e.g., postlunch was a 2 × 2 × 4 factorial ANOVA to examine the effect of exercise, metformin, and time, respectively). Insulin, glucagon, glucose, lactate, and NEFAs were log transformed before the statistical analyses to favor normality of residuals and homogeneity of variance. The nontransformed mean ± standard deviations data are presented. Statistical tests were two-tailed, and P values of ≤0.05 were considered significant. Statistical analyses were performed with SPSS 18 (SPSS Inc., Chicago, IL).
RESULTS
Baseline characteristics are presented in Table 1. Some reported mild to moderate gastrointestinal side effects during the metformin intervention; but all participants except one (final metformin dosage, 1,500 mg/day) were able to tolerate the maximum dosage of 2,000 mg/day during the last week of the intervention. Half of the participants started with 28 days of metformin.
Table 1.
Participant characteristics
| Baseline | |
|---|---|
| Sex (men/postmenopausal women) | 8/2 |
| Age (years) | 58 ± 6 |
| BMI (kg/m2) | 28.6 ± 5.3 |
| Weight (kg) | 86.9 ± 18.7 |
| A1C (%) | 6.5 ± 0.6 |
| Fasting glucose (mmol/L) | 7.3 ± 0.6 |
| Vo2peak (mL · kg−1 · min−1) | 30.2 ± 5.1 |
Data are reported as mean ± SD.
Oxygen consumption (Vo2), respiratory exchange ratio (RER), HR, and rating of perceived exertion (RPE) during exercise are shown in Table 2. There was a main effect of exercise intensity (all P ≤ 0.01) and no interaction effect (all P ≥ 0.18) for Vo2, RER, HR, and RPE. The first 15-min bout was performed at 33.9 ± 5.4% of Vo2peak, the second 15-min bout averaged 67.2 ± 7.3% of Vo2peak, and the third 5-min bout averaged 79.4 ± 8.8% of Vo2peak. Vo2 was not affected by metformin (P = 0.60). However, mean RER was lower in the metformin condition (0.96 ± 0.02 vs. 0.98 ± 0.02; P = 0.03). Mean HR was significantly higher in the metformin condition (124 ± 9 vs. 118 ± 8 beats per minute [bpm]; P = 0.009). The mean subjective ratings of perceived exertion during exercise were similar in the metformin and placebo conditions. However, participants reported a higher perceived exertion on their first exercise day regardless of whether they were on metformin or placebo. As well, when considering treatment order in the analyses, RPE was higher in the metformin condition (P = 0.03).
Table 2.
Effect of metformin on exercise-related outcomes
| Metformin | Placebo | Metformin vs. placebo conditions | Low vs. moderate vs. vigorous intensity | Interaction (condition × intensity) | |
|---|---|---|---|---|---|
| Treadmill exercise | |||||
| Vo2 (mL · min−1 · kg−1) | P = 0.60 | P < 0.01 | P = 0.18 | ||
| Low intensity | 10.09 ± 1.01 | 10.01 ± 1.62 | |||
| Moderate intensity | 20.45 ± 3.69 | 20.02 ± 3.74 | |||
| Vigorous intensity | 23.95 ± 4.01 | 23.81 ± 4.37 | |||
| RER | P = 0.03 | P < 0.01 | P = 0.38 | ||
| Low intensity | 0.92 ± 0.06 | 0.95 ± 0.08 | |||
| Moderate intensity | 0.96 ± 0.03 | 0.97 ± 0.03 | |||
| Vigorous intensity | 0.99 ± 0.08 | 1.02 ± 0.07 | |||
| HR (bpm) | P = 0.01 | P < 0.01 | P = 0.23 | ||
| Low intensity | 97 ± 12 | 91 ± 13 | |||
| Moderate intensity | 132 ± 21 | 126 ± 19 | |||
| Vigorous intensity | 142 ± 24 | 138 ± 21 | |||
| RPE (Borg) | P = 0.03 | P < 0.01 | P = 0.62 | ||
| Low intensity | 8 ± 1 | 8 ± 1 | |||
| Moderate intensity | 13 ± 1 | 13 ± 1 | |||
| Vigorous intensity | 14 ± 1 | 13 ± 1 | |||
| Isokinetic strength | |||||
| Peak torque | 70 ± 25 | 74 ± 26 | P = 0.13 | — | — |
| Mean torque | 58 ± 21 | 60 ± 23 | P = 0.12 | — | — |
| Fatigue index | 33 ± 10 | 36 ± 11 | P = 0.32 | — | — |
Data are reported as mean ± SD. RER is in Vco2/Vo2; RPE is on a scale of 6–20. Analyses were adjusted for treatment order (i.e., metformin first vs. placebo first).
Plasma metformin concentrations
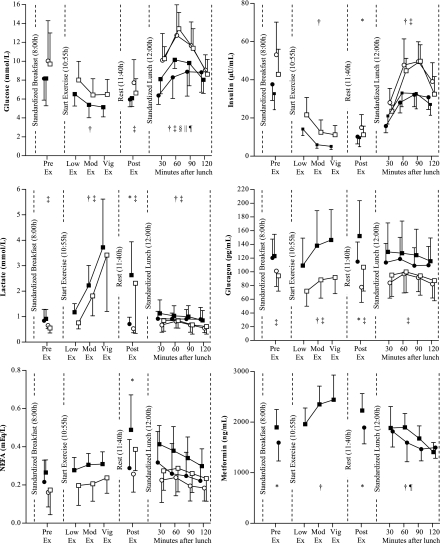
Plasma metformin concentrations were higher on the exercise day compared with the nonexercise day 25 min before exercise (1,897 ± 352 vs. 1,594 ± 363 ng/mL; P = 0.02) and 20 min after exercise (2,230 ± 335 vs. 1,893 ± 323 ng/mL; P = 0.01). Plasma metformin concentrations showed a significant time by exercise interaction (P = 0.05) during the 2-h postmeal period, with metformin concentration becoming similar near the end of the 2-h period.
Plasma glucose, lactate, NEFAs, insulin, and glucagon
Fasting glucose was lower in the metformin condition compared with the placebo condition (6.4 ± 0.6 vs. 7.2 ± 0.6 mmol/L; P = 0.02). As shown in Fig. 1, the mean glucose concentration continued to be lower throughout the day in the metformin conditions compared with placebo, with the difference becoming statistically significant after exercise. According to the sample taken immediately before lunch, exercise lowered plasma glucose concentration in the placebo condition but not in the metformin condition (−1.1 ± 2.0 vs. 0.1 ± 1.1 mmol/L, respectively); however, the metformin × exercise interaction was not significant (P = 0.17). The metformin × exercise interaction reached statistical significance during the 2-h postlunch period (P = 0.05), suggesting that exercise caused an increased glycemic response in the metformin condition but not in the placebo condition.
Figure 1.
Data are reported as mean ± SD (except for insulin: mean ± SEM). Analyses were adjusted for treatment order (i.e., metformin first vs. placebo first). N = 9 or 10. NA, not applicable; Mod, moderate; Vig, vigorous; Ex, exercise. ●, metformin + no exercise; ■, metformin + exercise; ○, placebo + no exercise; □, placebo + exercise. *Effect of exercise; †effect of time; ‡effect of metformin; §exercise by metformin interaction; ||exercise by time interaction; ¶metformin by time interaction.
Throughout the entire sessions, lactate concentrations were higher in the metformin condition compared with placebo (all P ≤ 0.05). Lactate concentrations increased with increasing exercise intensity and remained elevated for 20 min after exercise (both P < 0.01). NEFA concentrations were also increased after exercise (P < 0.01).
Insulin concentrations were lowered by exercise but were similar in the metformin versus placebo conditions throughout the day with the exception of higher insulin concentrations after lunch in the placebo condition (P < 0.01). Glucagon concentrations were increased in the metformin conditions (all P < 0.01).
Potential confounding variables
There were no significant differences in total calorie intake (1,906 ± 293 vs. 1,978 ± 518 kcal; P = 0.51) or distribution of macronutrients in the 24 h preceding the testing sessions (all P > 0.52). There was no difference in the amount of physical activity completed during the metformin versus placebo conditions (Godin Leisure Time Questionnaire score 36 ± 21 vs. 52 ± 36; P = 0.13). Participants did not report experiencing any difference in symptoms such as abdominal discomfort between conditions. Participants rated a higher likelihood of taking metformin while they were in the metformin condition compared with placebo (89 ± 40 mm vs. 62 ± 47 mm on the 150-mm visual analog scale), but the difference did not reach statistical significance (P = 0.20). Body mass was similar after the 28-day placebo condition versus after the 28-day metformin condition (86.7 ± 19.0 vs. 86.7 ± 18.9 kg; P = 0.93).
CONCLUSIONS
In the 1960s and 1970s several studies had investigated the effect of metformin on exercise performance because of concerns over lactic acidosis (see reference 13 for a detailed review). Although the combination of metformin and exercise was perceived as safe, interest in this area has reemerged in recent years (4,7,14,15). The current study is unique in that it focused on continuous exercise at several submaximal intensities that are relevant to activity patterns of people with type 2 diabetes and that we examined the interaction between exercise and metformin on the glycemic and hormonal responses to a subsequent meal.
We found that metformin increased lipid oxidation as evidenced by a lower RER during all three submaximal intensities of exercise. According to nonprotein RER tables, this would correspond to an increased lipid oxidation from 16 to 26% of total energy expenditure when walking at 3.5 km/h. Increased lipid oxidation is considered a normal adaption to exercise training. However, metformin increased submaximal HR and lactate concentrations, which are opposite to the direction of changes expected with regular exercise training. In the current study, HR was increased by a mean of 6 bpm. Interestingly, Sharoff et al. (4) also found an increased HR of about 8 and 5 bpm during exercise at 65 and 85% of Vo2peak, respectively; however, in their study the increase in HR did not reach statistical significance. In our study, a higher rating of perceived exertion in the metformin condition was also observed, although participants were all able to complete the exercise bouts. Taken together, this suggests that metformin has the potential to lower some patients’ selected exercise intensity since perceived exertion and HR are common feedback modalities and are frequently used to prescribe exercise intensities.
Although statistical significance was not reached, peak and mean torque for knee extension were lower in the metformin condition. Lower mean torque may have been expected based on the reduced muscle ATP concentrations observed by week 4 of metformin treatment in the study by Musi et al. (16).
Maximal metformin concentrations are typically reached 120–240 min after a dose. In the current study, we observed greater plasma metformin concentrations 25 min before exercise and 20 min after exercise compared with samples taken at the same times on the rest day (150 and 225 min postdose). The reasons for these higher concentrations are unknown but may have been caused by the anticipatory and stress responses to exercise, which are known to increase HR and blood pressure while redistributing blood flow to tissues such as skeletal muscle (17). Hence, the alteration in blood flow may have caused a transient decrease in the distribution of drug to certain tissues, including the liver. Indeed, this may have contributed to the reduced hypoglycemic effect of metformin after exercise even though plasma concentrations were higher at some time points. A reduced renal blood flow could increase plasma concentrations of drugs such as metformin, which are primarily eliminated by the kidneys (18). This may have also contributed to some of the higher concentrations measured in the exercise group, although only a more complete assessment of plasma metformin concentrations and urinary recovery could answer this question. A limitation of the current study is that the three blood samples taken immediately after each aerobic exercise bout were not taken at the corresponding times on the nonexercise days.
Although some previous studies had suggested that the effects of exercise and metformin on insulin sensitivity (4) or on the risk of diabetes (3) are not additive, our results suggest that in some conditions the combination may in fact be less effective at lowering the glycemic response to a meal than metformin alone. The reasons for this are not clear, but may be related to the strong counterregulatory response when the two were combined. In our study, the glucagon concentrations peaked immediately before lunch and were highest in the combined metformin and exercise condition. In support of the notion that glucose production may have been increased by the higher glucagon concentrations, Sharoff et al. (4) showed that hepatic glucose production was increased 2 h after exercise with metformin, unchanged by metformin alone, and decreased by exercise alone (4). Important differences between our study and Sharoff et al. (4) are that in the latter study participants were nondiabetic and 2 to 3 weeks of metformin use did not appear to alter insulin sensitivity or resting glucose concentration. Nonetheless, taken together these studies provide interesting insight on glucose homeostasis after metformin and exercise.
The lack of improvement in postmeal (lunch) plasma glucose concentrations on the exercise days should not discourage the use of exercise as a treatment modality. Rather, this study emphasizes that it may be important to further consider the timing of exercise and meals to obtain optimal glycemic benefits. For example, others have shown that exercising in the fasting state (a condition that also leads to pronounced counterregulatory responses) was much less effective at lowering plasma glucose than was exercising after a meal (19,20).
Furthermore, it is important to remember that the exercise protocol in the current study ended with 5 min of exercise at an intensity above ventilatory threshold. Similarly, in the study by Sharoff et al. (4), the exercise protocol ended with 10 min at 85% of Vo2peak. High intensity exercise (i.e., above ventilatory threshold) in the postabsorptive state is known to cause an increase in counterregulatory hormones and glucose in type 2 diabetes (21). However, high intensity exercise performed 45 min after the beginning of breakfast led to a decreased glycemic response to a meal that was provided 2.5 h after exercise (22). It would be of interest to examine if interactions between metformin and exercise on glucose homeostasis would be as pronounced after lower intensity exercise.
Type 2 diabetes is characterized not only by insulin deficiency but also by hyperglucagonemia (23). We are aware of nonexercise studies that have suggested that metformin may increase glucagon concentrations, but the increases were not statistically significant (24,25). In the nonrandomized exercise studies by Cunha et al. (14,15), glucagon concentrations were significantly higher in the participants with type 2 diabetes taking metformin compared with those taking glibenclamide or those with normal glucose tolerance. Although speculative, the glucose-lowering benefits of metformin could be further enhanced by strategies that could help minimize the exercise-induced increased glucagon levels such as exercising after a meal.
In conclusion, our study reports several novel findings regarding the concomitant use of metformin and exercise, specifically: 1) increased HR during exercise with metformin, 2) higher plasma metformin concentrations with exercise, and 3) nonadditive effects of metformin and exercise on the glycemic response to feeding. In our opinion, the magnitudes of these effects were small but have the potential to reduce the effectiveness of this therapeutic combination in diabetes treatment. Additional research could help optimize the concurrent use of these important and widely prescribed treatment modalities for diabetes.
Acknowledgments
Funding for this study was provided by the Alberta Diabetes Institute and the University of Alberta EFF-SAS. R.Q.G. was supported by a studentship from the government of Egypt.
No potential conflicts of interest relevant to this article were reported.
N.G.B., C.R., and G.J.B. contributed to the design of the study, collected data, analyzed the blood samples, and analyzed data. S.T.J. contributed to the design of the study. R.C.B. contributed to the data analysis. R.Q.G. and D.R.B. analyzed the blood samples. All authors, including R.Z.L., contributed to discussion and reviewed and edited the manuscript.
Parts of this study were presented in abstract form at the Annual Scientific Conference of the Canadian Society for Exercise Physiology, Banff, Alberta, Canada, 15–18 October 2008.
The metformin and placebo were graciously provided by Apotex Inc. Canada.
The authors would like to thank the study participants for their time and efforts and to thank Dr. Jeffrey A. Johnson and Dr. Dean Eurich for their input; Dr. Ronald Dlin, Jason Howard, and Lindsay Hubenig for their assistance with data collection; and Scott Forbes, Ian MacLean, and Shirley Shostak for their assistance with the assays (all at the University of Alberta).
References
- 1.2009 Top 200 generic drugs by total prescriptions. Drug Topics 2010:1–3 [Google Scholar]
- 2.Nathan DM, Buse JB, Davidson MB, et al. Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2006;29:1963–1972 [DOI] [PubMed] [Google Scholar]
- 3.Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD, Vijay V; Indian Diabetes Prevention Programme (IDPP) The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia 2006;49:289–297 [DOI] [PubMed] [Google Scholar]
- 4.Sharoff CG, Hagobian TA, Malin SK, et al. Combining short-term metformin treatment and one bout of exercise does not increase insulin action in insulin-resistant individuals. Am J Physiol Endocrinol Metab 2010;298:E815–E823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou G, Myers R, Li Y, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 2001;108:1167–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Narkar VA, Downes M, Yu RT, et al. AMPK and PPARdelta agonists are exercise mimetics. Cell 2008;134:405–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jadhav S, Ferrell W, Greer IA, Petrie JR, Cobbe SM, Sattar N. Effects of metformin on microvascular function and exercise tolerance in women with angina and normal coronary arteries: a randomized, double-blind, placebo-controlled study. J Am Coll Cardiol 2006;48:956–963 [DOI] [PubMed] [Google Scholar]
- 8.Boulé NG, Weisnagel SJ, Lakka TA, et al. ; HERITAGE Family Study Effects of exercise training on glucose homeostasis: the HERITAGE Family Study. Diabetes Care 2005;28:108–114 [DOI] [PubMed] [Google Scholar]
- 9.American College of Sports Medicine; American Diabetes Association Exercise and type 2 diabetes: American College of Sports Medicine and the American Diabetes Association: joint position statement. Exercise and type 2 diabetes. Med Sci Sports Exerc 2010;42:2282–2303 [DOI] [PubMed] [Google Scholar]
- 10.Johnson ST, Tudor-Locke C, McCargar LJ, Bell RC. Measuring habitual walking speed of people with type 2 diabetes: are they meeting recommendations? Diabetes Care 2005;28:1503–1504 [DOI] [PubMed] [Google Scholar]
- 11.Gabr RQ, Padwal RS, Brocks DR. Determination of metformin in human plasma and urine by high-performance liquid chromatography using small sample volume and conventional octadecyl silane coloumn. J Pharm Pharm Sci 2010;13:486–494 [DOI] [PubMed] [Google Scholar]
- 12.Pereira MA, FitzerGerald SJ, Gregg EW, et al. A collection of Physical Activity Questionnaires for health-related research. Med Sci Sports Exerc 1997;29(Suppl.):S1–S205 [PubMed] [Google Scholar]
- 13.Johnson ST, Robert C, Bell GJ, Bell RC, Lewanczuk RZ, Boulé NG. Acute effect of metformin on exercise capacity in active males. Diabetes Obes Metab 2008;10:747–754 [DOI] [PubMed] [Google Scholar]
- 14.Cunha MR, da Silva ME, Machado HA, et al. The effects of metformin and glibenclamide on glucose metabolism, counter-regulatory hormones and cardiovascular responses in women with type 2 diabetes during exercise of moderate intensity. Diabet Med 2007;24:592–599 [DOI] [PubMed] [Google Scholar]
- 15.Cunha MR, Silva ME, Machado HA, et al. Cardiovascular, metabolic and hormonal responses to the progressive exercise performed to exhaustion in patients with type 2 diabetes treated with metformin or glyburide. Diabetes Obes Metab 2008;10:238–245 [DOI] [PubMed] [Google Scholar]
- 16.Musi N, Hirshman MF, Nygren J, et al. Metformin increases AMP-activated protein kinase activity in skeletal muscle of subjects with type 2 diabetes. Diabetes 2002;51:2074–2081 [DOI] [PubMed] [Google Scholar]
- 17.Armstrong RB, Hayes DA, Delp MD. Blood flow distribution in rat muscles during preexercise anticipatory response. J Appl Physiol 1989;67:1855–1861 [DOI] [PubMed] [Google Scholar]
- 18.Khazaeinia T, Ramsey AA, Tam YK. The effects of exercise on the pharmacokinetics of drugs. J Pharm Pharm Sci 2000;3:292–302 [PubMed] [Google Scholar]
- 19.Ferland A, Brassard P, Lemieux S, et al. Impact of high-fat/low-carbohydrate, high-, low-glycaemic index or low-caloric meals on glucose regulation during aerobic exercise in type 2 diabetes. Diabet Med 2009;26:589–595 [DOI] [PubMed] [Google Scholar]
- 20.Poirier P, Mawhinney S, Grondin L, et al. Prior meal enhances the plasma glucose lowering effect of exercise in type 2 diabetes. Med Sci Sports Exerc 2001;33:1259–1264 [DOI] [PubMed] [Google Scholar]
- 21.Kjaer M, Hollenbeck CB, Frey-Hewitt B, Galbo H, Haskell W, Reaven GM. Glucoregulation and hormonal responses to maximal exercise in non-insulin-dependent diabetes. J Appl Physiol 1990;68:2067–2074 [DOI] [PubMed] [Google Scholar]
- 22.Larsen JJ, Dela F, Madsbad S, Galbo H. The effect of intense exercise on postprandial glucose homeostasis in type II diabetic patients. Diabetologia 1999;42:1282–1292 [DOI] [PubMed] [Google Scholar]
- 23.Dunning BE, Gerich JE. The role of alpha-cell dysregulation in fasting and postprandial hyperglycemia in type 2 diabetes and therapeutic implications. Endocr Rev 2007;28:253–283 [DOI] [PubMed] [Google Scholar]
- 24.Jackson RA, Hawa MI, Jaspan JB, et al. Mechanism of metformin action in non-insulin-dependent diabetes. Diabetes 1987;36:632–640 [DOI] [PubMed] [Google Scholar]
- 25.Stumvoll M, Nurjhan N, Perriello G, Dailey G, Gerich JE. Metabolic effects of metformin in non-insulin-dependent diabetes mellitus. N Engl J Med 1995;333:550–554 [DOI] [PubMed] [Google Scholar]