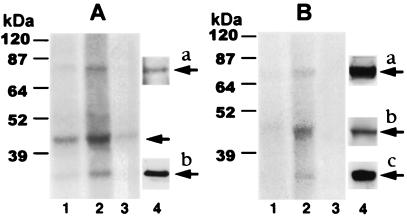
Figure 1.
Effect of chronic morphine on phosphorylation of proteins immunoprecipitated using anti-Gβ (A) or anti-GRK2/3 (B) antibodies. LMMP tissue obtained from opioid naïve (lane 1) and chronic morphine-treated (lane 2) guinea pigs was incubated with 32P for 2 h. Membranes were prepared, solubilized, and immunoprecipitated using antibodies selective for either Gβ (A) or GRK2/3 (B). In lane 3 of both panels, preadsorbed antiserum was used for immunoprecipitation of LMMP tissue obtained from chronic morphine-treated animals. Immunoprecipitates were subjected to SDS/PAGE, and radiolabeled proteins were visualized by their concomitant autoradiography (18-h exposure) using storage phosphorimaging techniques. Quantitative densitometric analysis was used to assess magnitude of 32P incorporation. Lane 4 in A shows Western analysis of GRK protein in Gβ immunoprecipitate (a) or purified Gβ protein (b). Lane 4a in B illustrates Western analysis of purified recombinant GRK2 protein. The presence of β-arrestin and Gβ proteins in GRK immunoprecipitate is illustrated in lanes 4b and 4c, respectively. Immunoblots were obtained as described in Materials and Methods. Figure is representative of four experiments. Molecular mass was assessed using a prestained protein ladder (10–200 kDa; Life Technologies) that was included in each run. Chemical identity of radiolabeled proteins was based on calculated molecular mass of autoradiographic signal, loss of signal following antibody preadsorption, and comparison of calculated molecular mass of signal obtained with autoradiographic vs. Western analysis. The apparent variability in the relative mobility of monitored protein in A vs. B results from variability in running time and other subtle aspects of SDS/PAGE among experiments. Protein molecular masses, calculated by extrapolation from the mobility of molecular mass standards, were very comparable among all experiments.