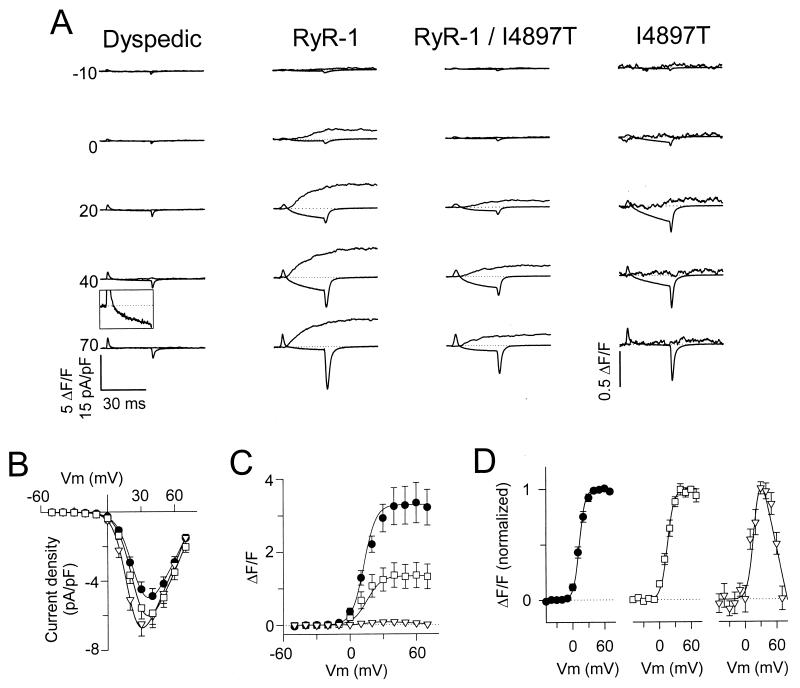
Figure 3.
The I4897T mutation in RyR1 disrupts voltage-gated SR Ca2+ release but not RyR1-mediated enhancement of DHPR channel activity. (A) Representative L-currents (lower traces) and Ca2+ transients (upper traces) obtained following 30-ms depolarizations to the indicated potentials obtained from uninjected dyspedic myotubes (first column) and dyspedic myotubes expressing RyR1 alone (second column), RyR1/I4897T (third column), or I4897T alone (fourth column). The outward deflection at the beginning of the current traces represents charge movement, which did not differ between uninjected and RyR1-expressing dyspedic myotubes (19, 20). For clarity, the ionic current during the voltage step to +40 mV for the uninjected dyspedic myotube (Inset) and all of the Ca2+ transients for the I4897T-expressing myotube were amplified ten times. Under these recording conditions (0.1 mM EGTA internal solution and 10-s interpulse duration), the baseline fluorescence was similar for all pulses within a sequence, even following pulses that elicit large Ca2+ release. (B–D) Average voltage dependence of peak L-current density (B) and Ca2+ transients (C) for dyspedic myotubes expressing RyR1 alone (black circles), RyR1/I4897T (white squares), or I4897T alone (white triangles). (D) Voltage dependence of the Ca2+ transients normalized to their respective peak values. The U-shaped curve through the data for the I4897T-expressing myotubes was obtained by inverting, and normalizing to one, the I–V curve shown in B.