Abstract
Background
p16 Methylation is a potential biomarker for prediction of malignant transformation of epithelial dysplasia. A probe-based, quantitative, methylation-specific PCR (MSP) called MethyLight may become an eligible method for detecting this marker clinically. We studied oral mucosa biopsies with epithelial dysplasia from 78 patients enrolled in a published 4-years' followup cohort, in which cancer risk for patients with p16 methylation-positive dysplasia was significantly higher than those without p16 methylation (by 150-bp MSP and bisulfite sequencing; +133 ~ +283, transcription starting site, +1). The p16 methylation status in samples (N = 102) containing sufficient DNA was analyzed by the 70-bp classic (+238 ~ +307) and 115-bp novel (+157 ~ +272) MethyLight assays, respectively.
Results
p16 Methylation was detectable in 75 samples using the classic MethyLight assay. The methylated-p16 positive rate and proportion of methylated-p16 by the MethyLight in MSP-positive samples were higher than those in MSP-negative samples (positive rate: 37/44 vs. 38/58, P=0.035, two-sided; proportion [median]: 0.78 vs. 0.02, P <0.007). Using the published results of MSP as a golden standard, we found sensitivity, specificity, and accuracy for this MethyLight assay to be 70.5%, 84.5%, and 55.0%, respectively. Because amplicon of the classic MethyLight procedure only partially overlapped with the MSP amplicon, we further designed a 115-bp novel MethyLight assay in which the amplicon on the sense-strand fully overlapped with the MSP amplicon on the antisense-strand. Using the 115-bp MethyLight assay, we observed methylated-p16 in 26 of 44 MSP-positive samples and 2 of 58 MSP-negative ones (P = 0.000). These results were confirmed with clone sequencing. Sensitivity, specificity, and accuracy using the 115-bp MethyLight assay were 59.1%, 98.3%, and 57.4%, respectively. Significant differences in the oral cancer rate were observed during the followup between patients (≥60 years) with and without methylated-p16 as detected by the 115-bp MethyLight assay (6/8 vs. 6/22, P = 0.034, two-sided).
Conclusions
The 115-bp MethyLight assay is a useful and practical assay with very high specificity for the detection of p16 methylation clinically.
Background
Aberrant methylation of CpG islands is a very stable modification of genomic DNA that often inactivates gene expression pathologically. Methylation of a target CpG island in even 0.1% of a cell population obtained from fixed/frozen tissues or body fluids can be detected readily. The high stability and high sensitivity of detection make DNA methylation one kind of optimal clinical biomarker for the prediction of potential malignancy progression of precancerous lesions, metastasis/recurrence of cancer, and chemo/radio-therapy sensitivity [1].
It is well recognized that complete methylation of CpG sites within CpG islands around transcription start sites represents deep-silencing of gene expression established during embryo development and cell differentiation. Well-documented examples include the silencing of tissue-specific genes, gene imprinting, inactivation of parasite DNA and X-chromosome. However, the methylation of CpG islands in tumor suppressor genes, including p16, is a progressive process encountered during carcinogenesis [2-4]. De novo methylation often occurs post gene silencing at a few seeding CpG sites in initiation and precancerous stages, and ultimately extends to the full CpG island in advanced cancer. This complicates the development of an assay to detect the methylation status of a target CpG island in which complete methylation is not established. For example, methylation of crucial CpG sites within a CpG island that correlates with clinical outcomes should first be identified, and then a proper detection approach with high specificity for clinical diagnosis should be designed. Unfortunately, such crucial CpG sites are not well characterized for most CpG islands. This often leads to the dissimilar detection of methylation at different CpG sites within a target CpG island between different laboratories. Contradictory results often arise from different kinds of detection assays, or the same assay with different detection sensitivity [5].
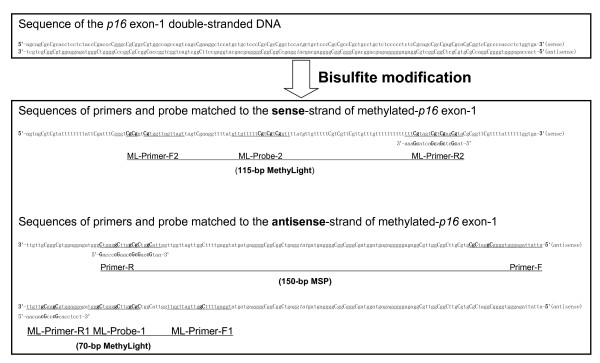
Tumor suppressor gene p16 (CDKN2A) controls cell proliferation through the P16-CDK4-RB pathway at the G1→S checkpoint of the cell cycle [6]. Frequent, aberrant methylation of a crucial CpG island is the main mechanism of inactivation for p16 in the early stages of carcinogenesis [1]. A number of nested case-control studies and followup cohorts consistently showed p16 methylation as a potential biomarker for the early prediction of malignant transformation of epithelial dysplasia, one kind of precancerous lesion in many organs/tissues including the oral/oesophageal/gastric mucosa [7-13]. Although bisulfite-clone sequencing provides detailed information about the methylation status of each CpG site in the cloning molecules, it is often used as a confirmation assay rather than a regular detection assay because of its low detection sensitivity (> 20%), labor, and time costs. A number of assays including MSP, MethyLight, Pyrosequencing, and DHPLC are often used to detect p16 methylation in laboratory research [3,7-16]. Among them, MethyLight, based on MSP primers, may become one of the most eligible, convenient, quantitative, and sensitive assays for the clinical detection of p16 methylation primarily because it uses a methylation-specific primer set and real-time, sequence-specific probe validation. In the present study, we evaluated the sensitivity, specificity, and accuracy of a 70-bp classic assay in which the amplicon partially overlapped with the MSP amplicon, and a 115-bp novel MethyLight assay in which the amplicon fully overlapped with the MSP amplicon (Figure 1). The data was collected from 102 oral epithelial dysplasia samples obtained from a followup cohort study, in which malignant transformation of this disease correlated with p16 methylation detected by MSP and was confirmed by clone sequencing [13].
Figure 1.
The sequence of the p16 exon-1 before and after bisulfite conversion. Locations of amplicons, primers, and probes used in the 150/151-bp MSP-m/u, 70-bp, and 115-bp MethyLight assays within the sense-strand or antisense-strand were underlined and labelled.
Results and Discussion
Detection of p16 methylation by a classic 70-bp MethyLight assay
An eligible PCR-based molecular assay for diagnosis should meet several essential requirements including high specificity, real-time validation using a sequence-specific probe, positive confirmation with direct sequencing, and refractory to carry-over contamination. Combination of MethyLight using methylation-specific primers with probes containing an anti-contamination system, composed replacing dTTP with dUTP and the addition of a uracil glycosylase UNG in the PCR reaction mixture, may become an ideal method for the clinical detection of methylation in a specific CpG island. In a 4-year followup cohort, we reported that methylated-p16 was a potential biomarker for early prediction of malignant transformation of oral epithelial dysplasia [13]. Among patients of at least 60 years of age, the sensitivity and specificity of methylated-p16 were 77% and 78%, respectively. Hall et al. reported similar results [14]. Therefore, the using MethyLight as a clinical assay to detect methylated-p16 was feasible.
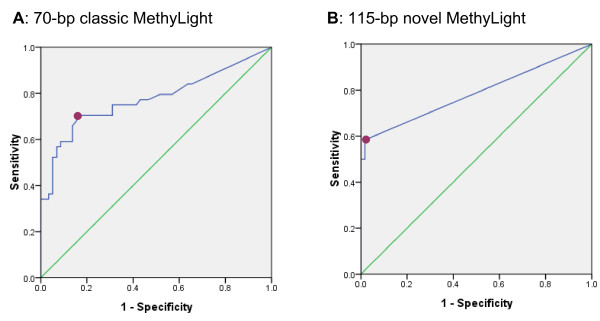
The 70-bp classic MethyLight for methylated-p16 was evaluated using either genomic DNA of baseline or followup samples (n = 102) from patients enrolled in the mentioned cohort (n = 78). After genomic DNA was converted to SafeBis templates as described in the methods section, the methylated-p16 was analyzed with the classic MethyLight. Methylated-p16 was detected in 75 of 102 tested samples. The methylated-p16 MethyLight-positive rate and proportion of methylated-p16 in 44 methylated-p16 MSP-positive samples were higher than those in 58 MSP-negative samples, respectively (positive rate: 37/44 vs. 38/58, P = 0.035, two-sided; proportion [median]: 0.78 vs. 0.02, P <0.007). Using the prognosis-related MSP-results of methylated-p16 as a golden standard, we found sensitivity, specificity, and accuracy for the classic MethyLight were 70.5%, 84.5%, and 55.0% with a cut-off point of RCN set at 0.073, respectively (Figure 2A).
Figure 2.
ROC curves of detection of methylated-p16 by two MethyLight assays. The sensitivity and specificity of the MethyLight assay at various points of relative copy number in 102 tested samples were calculated according to the result of the 150-bp MSP. The cut-off points of RCN were marked by the deep-red circlets. A: For the 70-bp classic MethyLight, the area under the curve is 0.776 (95% CI: 0.677-0.874), P = 0.000. When the cut-off point of RCN was 0.0725, the sensitivity and specificity were 0.705 and 0.845, respectively. B: For the 115-bp novel MethyLight assay, the area under the curve is 0.787 (95% CI: 0.689-0.884), P = 0.000. When the cut-off point of RCN was 0.0002, the sensitivity and specificity were 0.591 and 0.983, respectively.
Development of a 115-bp novel MethyLight assay
After conversion of unmethylated cytosine residues to uracil (or thymine in PCR products; C → U/T) residues, a double stranded DNA molecule is transformed into two non-complementary single-stranded DNA molecules (C≡G → U/T≠G), as illustrated in Figure 1. Interestingly, all current methylation detection assays for the p16 CpG islands are designed according to the antisense-strand sequence of the p16 exon-1, while none target the sense-strand. The main reasons may include the good performance of first 150/151-bp MSP-m/u for methylated/unmethylated-p16 in cell line and tissue samples, and the very high content (111/175) of thymine residues in the unmethylated sense-strand present after bisulfite modification, which makes it difficult to design a proper unmethylation-specific primer set that can be used as control MSP-u in the case that p-16 is not methylated (Figure 1). However, in the MethyLight assay, instead of using the template corresponding to unmethylated p-16, the COL2A1 gene, without a CpG island, is recommended as an optimal common reference for all tested CpG islands for quantification of modified genomic DNA in the tested samples [17]. Using this strategy, the sense-strand of the methylated-p16 can be used to design a MethyLight assay.
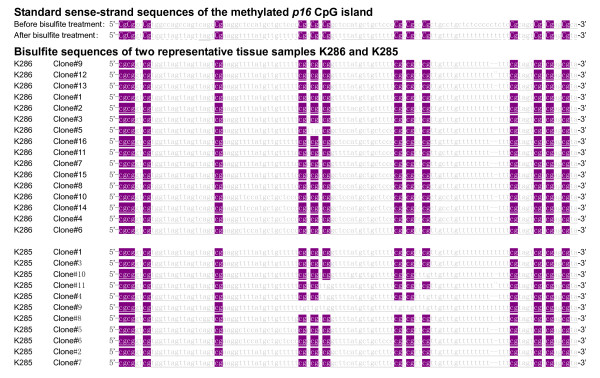
The amplicon (+238 ~ +307; transcription starting site, +1) of the 70-bp MethyLight partially overlapped with the 150-bp MSP amplicon (+133 ~ +283) (Figure 1). To investigate the feasibility of using the p16 exon-1 sense-strand for detection of methylated-p16, we designed a 115-bp novel MethyLight assay according to the sense-strand believing it might correlate with the 150-bp MSP better than the 70-bp MethyLight assay. This theory was based on the fact that the 115-bp MethyLight amplicon matched to the 150-bp MSP amplicon better than the 70-bp MethyLight amplicon (Figure 1). Using the novel MethyLight assay, we observed methylated-p16 in 26 of 44 MSP-positive samples and 2 of 58 MSP-negative ones (P=0.000). This result was confirmed by clone sequencing two representative samples (Figure 3). When the RCN cut-off point was set at 0.0002, the sensitivity, specificity, and accuracy of the 115-bp MethyLight were 59.1%, 98.3%, and 57.4%, respectively (Figure 2B).
Figure 3.
Results of clone sequencing of the 115-bp MethyLight PCR products. The sense-strand sequences of the methylated p16 CpG island with and without bisulfite treatment were listed. The bisulfite-treated template of two 115-bp MethyLight-positive samples was amplified with the same primer set. The PCR products of these two representative samples were clone-sequenced, respectively. 99.6% (223/224) and 93.5% (144/154) cytosines at CpG sites within total 16 and 11 clones (14 CpG sites/clone) from the sample K286 and K285 were maintained and 77.8% (548/704) and 90.9% (440/484) cytosines at non-CpG sites within these clones (44 non-CpG sites/clone) were converted to thymines, respectively. These results indicate that these clones are fully methylated at all CpG sites.
Comparison of two MethyLight assays
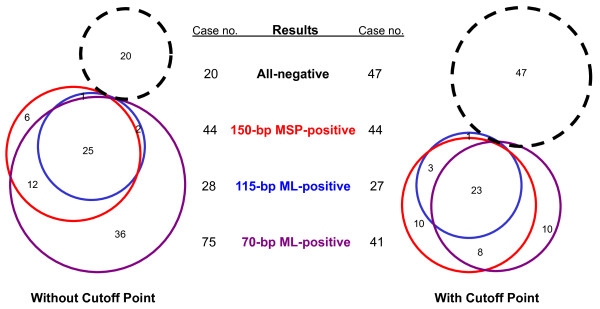
Furthermore, we compared the results of two MethyLight assays and found 23 of the 41 (56.1%) classic MethyLight positive samples are also 115-bp MethyLight positive; whereas only 4 of 61 (6.6%) classic MethyLight negative samples are 115-bp MethyLight positive (P = 0.000). The detailed overlap information for the results of methylated-p16 in all 102 tested samples was analyzed (Figure 4). Apparently, when the RCN cut-off points were set for the two MethyLight assays, the novel and classic assays had the similar accuracy (57% and 55%). However, the 115-bp MethyLight had a very high specificity, while the classic MethyLight had a higher sensitivity. Most importantly, the sensitivity and specificity of the novel MethyLight assay are consistent regardless of whether the RCN cut-off point was used during the calculation process. This indicates the 115-bp assay could be used as a qualitative assay for clinical detection of methylated-p16.
Figure 4.
Overlapping information of methylated-p16 by different assays. 102 oral mucosa biopsy samples were tested using three assays; red circles indicate methylated-p16 positive sample numbers as determined using the 150-bp MSP; blue circles indicate methylated-p16 positive sample numbers as determined using the 115-bp novel MethyLight assay; violet circles indicate methylated-p16 positive sample numbers as determined using the 70-bp classic MethyLight assay; the black dashed line circles represent samples without methylated-p16 detected using the three assays; the number within each open area covered by different cycles represents the exact number of samples containing methylated-p16 as detected by the corresponding assays. A: Qualitative results without the use of a cut-off point. B: Summary of the total number of samples with or without methylated-p16 as determined by each assay (left/right case no., without/with cut-off point). C: Qualitative results using a cut-off point for the two MethyLight assays (relative copy number; 0.073 for the classic MethyLight assay, and 0.0002 for the novel MethyLight assay).
We further analyzed the clinical outcome of methylated-p16 as detected by two MethyLight assays. Among 30 patients of at least 60 years of age, methylated-p16 was detected in 8 baseline samples by the 115-bp MethyLight assay (with or without the cut-off value). During the followup period, oral cancer developed in 6 of 8 methylated-p16 positive patients (75.0%), but only 6 of 22 patients (27.3%) without methylated-p16 developed oral cancer [odd ratio 8.00 (95% CI, 0.98~80.93; P = 0.034, two-sided). Among 34 patients analyzed using the classic MethyLight assay (with cut-off value 0.073), the odds ratio of methylated-p16 was 3.64 (6/10 vs. 7/24; 95% CI, 0.62~21.91; P = 0.130). These results suggest that the 115-bp MethyLight assay might be better suited to detect the methylated-p16 biomarker than the classic MethyLight assay.
Conclusions
The 115-bp MethyLight assay maybe a practical assay for the detection of methylated-p16 biomarker for clinical diagnosis.
Methods
Patients and oral biopsies
102 genomic DNA samples (> 500 ng) were extracted from paraffin-embedded oral mucosa biopsies containing mild or moderate dysplasia lesions from 78 patients enrolled in a 4-year follow-up cohort (NCT00835341, available at http://ClinicalTrials.gov) [7,13]. Briefly, the fixed tissue block was cut into 10 μm slides, treated with xylene to remove the paraffin, rehydrated with graded ethanol, mixed with lysis buffer containing 100 μg proteinase K, digested at 56°C overnight, and incubated 10 min at 95°C to stop the digestion [18]. DNA present in the digestion solution was precipitated with ethanol and dissolved in 50 μl TE buffer. DNA concentration was determined spectrophotometrically with diphenylamine as described [19]. The average recovery rate of genomic DNA was 77.6%. 61 samples were baseline biopsies and the remaining 41 samples were taken during the followup periods. Methylation status of the antisense-strand of exon-1 within the p16 CpG island was determined using a 150-bp MSP assay in which DHPLC was used as the detector; the results were further confirmed through clone sequencing (Figure 1). Methylated-p16 was detected in 44 of these samples. The study was approved by the Institutional Review Boards of Peking University School of Stomatology and School of Oncology, and all patients gave written informed consent.
Preparation of SafeBis DNA by bisulfite treatment
Genomic DNA samples (2 μg) were treated with bisulfite for 16 hrs at 50°C without desulfonation as described [20], purified with the Wizard DNA Clean-Up System Kit (Promega, Madison, WI), dissolved in 40 μl TE preheated to 80°C, and stored in three aliquots at -20°C before use. The unmethylated cytosine residues in the DNA were converted to uracil (thymine in PCR products) and the methylated cytosine residues remained intact after this treatment.
Detection of p16 methylation by the 70-bp classic MethyLight assay
Methylation of CpG sites across the MSP Primer-R region in the antisense-strand of the p16 exon-1 was analyzed by the classic MethyLight assay using modified primers [15]. Briefly, the ML-Primer-F1 (5'-tggag ttttC ggttg attgg tt-3'), ML-Primer-R1 (5'-aacaa cGccc Gcacc tcct-3'), and a methylated-p16-specific ML-Probe-1 (6FAM5'-accCg acccC gaacC gCg-3'TAMRA, TaqMan) were used to detect the 70-bp methylated p16 templates in the SafeBis DNA (Figure 1). The reference gene COL2A1 was also amplified with a forward primer (5'-tctaa caatt ataaa ctcca accac caa-3'), a reverse primer (5'-gggaa gatgg gatag aaggg aatat-3'), and a COL2A1-specific probe (6FAM5'-ccttc attct aaccc aatac ctatc ccacc tctaa a-3'BHQ1) [17]. A uracil DNA glycosylase (UNG) carry-over prevention system was employed in the MethyLight assay [18]. The 20 μl MethyLight reaction mixture contained 2 μl 10×PCR buffer (Qiagen, Germany), 0.5 units of HotStar Taq DNA polymerase (Qiagen), 200 μmol/L dATP, 200 μmol/L dCTP, 200 μmol/L dGTP, 800 μmol/L dUTP (Promaga), 5 mmol/L MgCl2, 75 nmol/L of each primer (TaKaRa, Beijing), 75 nmol/L probe (TaKaRa), 2 μl 10×UNG Buffer (NEB), 0.4 units UNG (NEB), and 10 ng template. An ABI7500 thermal cycler was used to conduct the PCR reactions using the following thermal conditions: 37°C for 10 min → 95°C for 30 min → (95°C for 15 sec → 62°C for 1 min) × 45 cycles. The fluorescence value was detected at 62°C. Duplicate tubes were used for each sample, and the average Ct value was used in the calculations. Relative copy number (RCN) of methylated-p16 was calculated according to the formula [2-ΔCt, (ΔCt = Ctmethylated-p16 - CtCOL2A1)]. RKO and MGC803 xenografts from nude mice were also used as methylated-p16 positive and negative controls in each experiment, respectively [13]. The calculated RCN of methylated-p16 in each sample was standardized according to the RCN of RKO positive control.
Detection of p16 methylation by the 115-bp MethyLight assay
The ML-Primer-F2 (5'-CgCgg tCgtg gttag ttagt-3'), ML-Primer-R2 (5'-tacGc tcGac Gacta Cgaaa-3'), and ML-Probe-2 (5'-6FAM-gttgt ttttC gtCgt Cggtt-TAMRA-3') were used to detect the 115-bp methylated fragment of the sense-strand of p16 exon-1, which completely overlapped the sense-strand sequence corresponding to the 150-bp MSP amplicon within the antisense-strand (Figure 1). Other conditions were the same as the classic MethyLight assay.
Clone sequencing of the 115-bp MethyLight PCR products of methylated-p16
The SafeBis template from two representative samples of the 115-bp MethyLight-positive samples was amplified with the same primer set used in the 115-bp MethyLight assay (without the ML-Probe-2), and then clone-sequenced as described [3].
Statistical methods
A ROC curve of the results for each MethyLight assay was calculated. Results of methylated-p16 in these tested samples, determined using the 150-bp MSP-m (and by151-bp MSP-u in the MSP-m negative cases), were used as the golden standard in the calculation of sensitivity and specificity for the two MethyLight assays (Figure 1). These results showed a strong correlation with the malignant transformation of these lesions in the 4-year followup cohort study [13]. The accuracy was calculated according to the formula [Sensitivity+Specificity-1]. The Chi-square test and Student's t-test were used to test the significance of qualitative and quantitative data between different groups. All tests were two-sided.
Authors' contributions
JZ carried out the molecular epigenetic assays. ZL carried out the clone sequencing. JC and HL collected the tested samples. DD conceived the study and drafted the manuscript. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Jing Zhou, Email: jane72@163.com.
Jie Cao, Email: dr.caojie@gmail.com.
Zheming Lu, Email: zheminglu@163.com.
Hongwei Liu, Email: hongweil@bjmu.edu.cn.
Dajun Deng, Email: dengdajun@bjmu.edu.cn.
Acknowledgements
This work is supported by Capital Program for Development of Health Science (Grant #434), Beijing Science and Technology Commission (Grant #Z090507017709016), Key Technologies Research & Development Programs (863 Grants #2006AA020902 and 2006AA02A402). We thank Dr. Zhaojun Liu for preparation of ROC curve charts. We also thank Professor Huidong Shi and Mr. James Wilson (Augusta, Georgia) for language editing.
References
- Deng D, Liu Z, Du Y. Epigenetic alterations as cancer diagnostic, prognostic, and predictive biomarkers. Adv Genet. 2010;71:125–176. doi: 10.1016/B978-0-12-380864-6.00005-5. [DOI] [PubMed] [Google Scholar]
- Wong DJ, Foster SA, Galloway DA, Reid BJ. Progressive region-specific de novo methylation of the p16 CpG island in primary human mammary epithelial cell strains during escape from M(0) growth arrest. Mol Cell Biol. 1999;19:5642–5651. doi: 10.1128/mcb.19.8.5642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo DY, Zhang BZ, Lv LB, Xiang SY, Liu YH, Ji JF, Deng DJ. Methylation of CpG islands of p16 associated with progression of primary gastric carcinomas. Laboratory Investigation. 2006;86:591–598. doi: 10.1038/labinvest.3700415. [DOI] [PubMed] [Google Scholar]
- Hinshelwood RA, Melki JR, Huschtscha LI, Paul C, Song JZ, Stirzaker C, Reddel RR, Clark SJ. Aberrant de novo methylation of the p16INK4A CpG island is initiated post gene silencing in association with chromatin remodelling and mimics nucleosome positioning. Hum Mol Genet. 2009;18:3098–3109. doi: 10.1093/hmg/ddp251. [DOI] [PubMed] [Google Scholar]
- Capel E, Fléjou JF, Hamelin R. Assessment of MLH1 promoter methylation in relation to gene expression requires specific analysis. Oncogene. 2007;26:7596–7600. doi: 10.1038/sj.onc.1210581. [DOI] [PubMed] [Google Scholar]
- Serrano M, Hannon G, Beach D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature. 1993;366:704–707. doi: 10.1038/366704a0. [DOI] [PubMed] [Google Scholar]
- Sun Y, Deng DJ, You WC, Bai H, Zhang L, Zhou J, Shen L, Ma JL, Xie YQ, Li JY. Methylation of p16 CpG islands associated with malignant transformation of gastric dysplasia in a population-based study. Clinical Cancer Research. 2004;10:5087–5093. doi: 10.1158/1078-0432.CCR-03-0622. [DOI] [PubMed] [Google Scholar]
- Belinsky SA, Liechty KC, Gentry FD, Wolf HJ, Rogers J, Vu K, Haney J, Kenned TC, Hirsch FR, Miller Y, Franklin WA, Herman JG, Baylin SB, Bunn PA, Byers T. Promoter hypermethylation of multiple genes in sputum precedes lung cancer incidence in a high-risk cohort. Cancer Research. 2006;66:3338–3344. doi: 10.1158/0008-5472.CAN-05-3408. [DOI] [PubMed] [Google Scholar]
- Schulmann K, Sterian A, Berki A, Yin J, Sato F, Xu Y, Olaru A, Wang S, Mori Y, Deacu E, Hamilton J, Kan T, Krasna MJ, Beer DG, Pepe MS, Abraham JM, Feng Z, Schmiegel W, Greenwald BD, Meltzer SJ. Inactivation of p16, RUNX3, and HPP1 occurs early in Barrett's-associated neoplastic progression and predicts progression risk. Oncogene. 2005;24:4138–4148. doi: 10.1038/sj.onc.1208598. [DOI] [PubMed] [Google Scholar]
- Wang JS, Guo M, Montgomery EA, Thompson RE, Cosby H, Hicks L, Wang S, Herman JG, Canto MI. DNA promoter hypermethylation of p16 and APC predicts neoplastic progression in Barrett's esophagus. Am J Gastroenterol. 2009;104:2153–2160. doi: 10.1038/ajg.2009.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Z, Cheng Y, Gu W, Zheng Y, Sato F, Mori Y, Olaru A, Paun B, Yang J, Kan T, Ito T, Hamilton JP, Selaru FM, Agarwal R, David S, Abraham JM, Wolfsen HC, Wallace MB, Shaheen NJ, Washington K, Wang J, Canto MI, Bhattacharyya A, Nelson MA, Wagner PD, Romero Y, Wang KK, Feng Z, Sampliner RE, Meltzer SJ. A multicenter, double-blinded validation study of methylation biomarkers for progression prediction in Barrett's esophagus. Cancer Res. 2009;69:4112–4115. doi: 10.1158/0008-5472.CAN-09-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall G, Shaw R, Field E, Rogers S, Sutton D, Woolgar J, Lowe D, Liloglou T, Field J, Risk J. p16 Promoter methylation is a potential predictor of malignant transformation in oral epithelial dysplasia. Cancer Epidemiol Biomarkers Prev. 2008;17:2174–2179. doi: 10.1158/1055-9965.EPI-07-2867. [DOI] [PubMed] [Google Scholar]
- Cao J, Zhou J, Gao Y, Gu LK, Meng HX, Liu HW, Deng DJ. Methylation of p16 CpG Island Associated with Malignant Progression of Oral Epithelial Dysplasia: A Prospective Cohort Study. Clinical Cancer Research. 2009;15:5178–5183. doi: 10.1158/1078-0432.CCR-09-0580. [DOI] [PubMed] [Google Scholar]
- Herman JG, Graff JR, Myohanen S, Nelkin BD, Baylin SB. Methylation-specific PCR: A novel PCR assay for methylation status of CpG islands. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:9821–9826. doi: 10.1073/pnas.93.18.9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eads CA, Lord RV, Kurumboor SK, Wickramasinghe K, Skinner ML, Long TI, Peters JH, DeMeester TR, Danenberg KD, Danenberg PV, Laird PW, Skinner KA. Fields of aberrant CpG island hypermethylation in Barrett's esophagus and associated adenocarcinoma. Cancer Res. 2000;60:5021–5026. [PubMed] [Google Scholar]
- Shaw RJ, Akufo-Tetteh EK, Risk JM, Field JK, Liloglou T. Methylation enrichment pyrosequencing: combining the specificity of MSP with validation by pyrosequencing. Nucleic Acids Res. 2006;34:e78. doi: 10.1093/nar/gkl424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widschwendter M, Siegmund KD, Müller HM, Fiegl H, Marth C, Müller-Holzner E, Jones PA, Laird PW. Association of breast cancer DNA methylation profiles with hormone receptor status and response to tamoxifen. Cancer Res. 2004;64:3807–3813. doi: 10.1158/0008-5472.CAN-03-3852. [DOI] [PubMed] [Google Scholar]
- Dieffenbach CW, Dveksler GS. PCR Primer: A Laboratory Manual. 1. New York: Cold Spring Harbor Laboratory Press; 1995. [Google Scholar]
- Furihata C, Yamawaki Y, Jin SS, Moriya H, Kodama K, Matsushima T, Ishikawa T, Takayama S, Nakadate M. Induction of unscheduled DNA synthesis in rat stomach mucosa by glandular stomach carcinogens. J Natl Cancer Inst. 1984;72:1327–1334. [PubMed] [Google Scholar]
- Tetzner R, Dietrich D, Distler J. Control of carry-over contamination for PCR-based DNA methylation quantification using bisulfite treated DNA. Nucleic Acids Res. 2007;35:e4. doi: 10.1093/nar/gkl955. [DOI] [PMC free article] [PubMed] [Google Scholar]