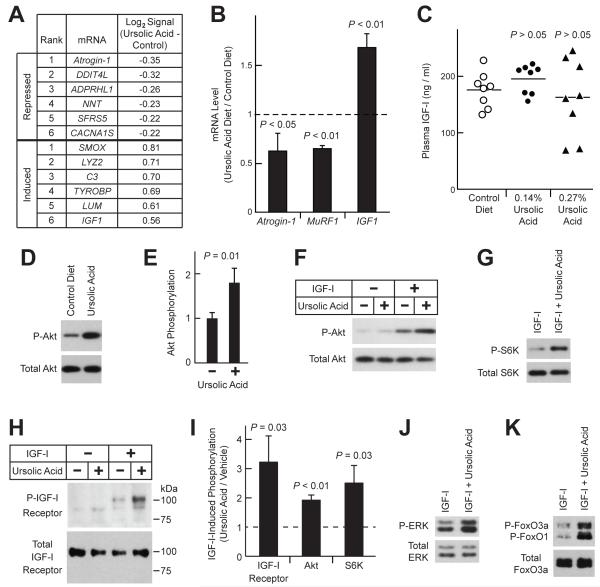
Figure 5. Ursolic Acid Promotes Muscle Growth by Repressing Atrophic Gene Expression, Inducing Trophic Gene Expression, and Enhancing Skeletal Muscle IGF-I Signaling.
(A-B) Mice were provided ad lib access to either standard chow (control diet) or standard chow supplemented with 0.27% ursolic acid (ursolic acid diet) for 5 weeks before tissues were harvested. (A) Ursolic acid-induced changes in the log2 hybridization signals of skeletal muscle mRNAs, as assessed by Affymetrix Mouse Exon 1.0 ST arrays. n = 4 arrays per diet; each array assessed gastrocnemius RNA pooled from two mice. Data were filtered for P ≤ 0.005 by unpaired t-test and log2 hybridization signal ≥ 8. Table shows the top 6 mRNAs most induced or repressed by dietary ursolic acid. (B) Effect of ursolic acid on IGF1, atrogin-1 and MuRF1 mRNA levels, as assessed by qPCR. Data are means ± SEM. (C) Mice were provided ad lib access to either standard chow (control diet) or standard chow supplemented with the indicated concentration of ursolic acid for 7 weeks before plasma IGF-I levels were measured. Each data point represents one mouse, and horizontal bars denote the means. P-values were determined by one-way ANOVA with Dunnett’s post-test. (D-E) Mice were provided ad lib access to either standard chow (control diet) or standard chow supplemented with 0.27% ursolic acid for 16 weeks. Total protein extracts from quadriceps muscles were subjected to SDS-PAGE, followed by immunoblot analysis for phosphorylated and total Akt, as indicated. (D) Representative immunoblot. (E) In each mouse, the level of phospho-Akt was normalized to the level of total Akt. These ratios were then normalized to the average phospho-Akt/total Akt ratio from control mice. Data are means ± SEM from 9 mice per diet. P-value was determined by unpaired t-test. (F-K) Serum-starved C2C12 myotubes were treated in the absence or presence of ursolic acid (10 μM) and/or IGF-I (10 nM), as indicated. For studies of the IGF-I receptor, cells were harvested 2 min later, and protein extracts were subjected to immunoprecipitation with anti-IGF-I receptor β antibody, followed by immunoblot analysis with anti-phospho-tyrosine or anti-IGF-I receptor β antibodies to assess phospho- and total IGF-I receptor, respectively. For other studies, cells were harvested 20 min after addition of ursolic acid and/or IGF-I, and immunoblot analyses were performed using total cellular protein extracts and antibodies specific for the phosphorylated or total proteins indicated. (F-H) Representative immunoblots showing effect of ursolic acid on IGF-I-mediated phosphorylation of Akt (F), S6K (G) and IGF-I receptor (H). (I) Quantification of the effect of ursolic acid on IGF-I-dependent phosphorylation of the IGF-I receptor, Akt and S6K. Levels in the presence of ursolic acid and IGF-I are normalized to levels in the presence of IGF-I alone, which were set at 1 and are indicated by the dashed line. Data are means ± SEM from ≥ 3 experiments. (J-K) Ursolic acid enhances IGF-I-mediated phosphorylation of ERK (J) and FoxO3a/FoxO1 (K). See also Table S4 and Figure S3.