Fig. 2.
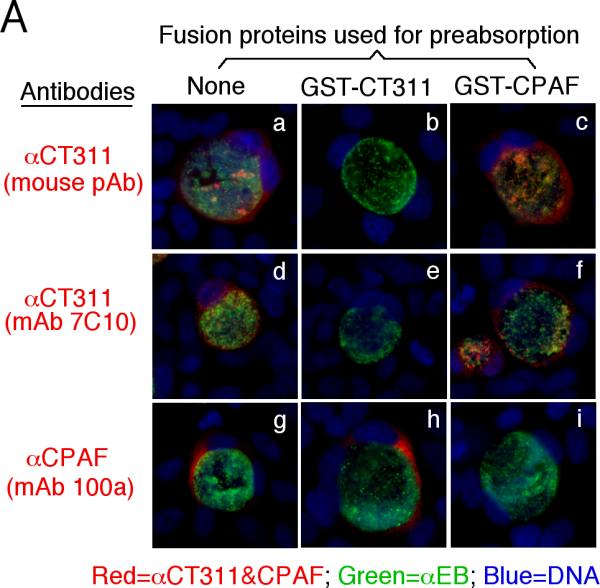
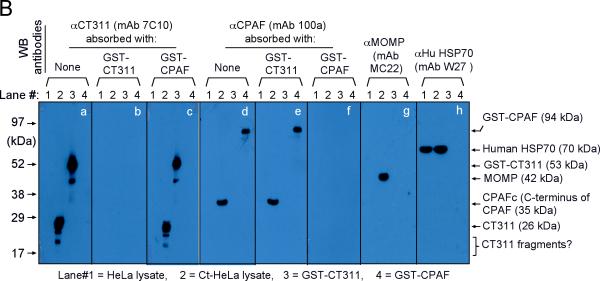
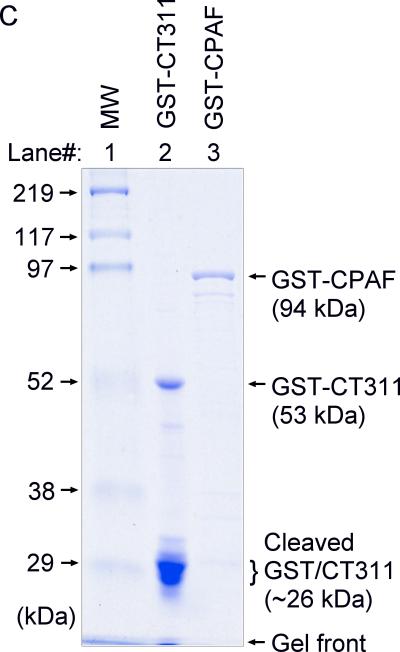
Specific detection of endogenous CT311 by the anti-CT311 antibodies. (A) The anti-CT311 mouse antiserum (panels a-c) and mAb 7C10 (d-f), the anti-CPAF mAb 100a (g-i) with (panels b, c, e, f, h & i) or without (a, d & g) pre-absorption with the corresponding or heterologous GST fusion proteins were used to detect the endogenous proteins in C. trachomatis-infected cells (red). Note that both the anti-CT311 and anti-CPAF antibody labelings were removed by pre-absorption with the corresponding (panels b, e & i) but not heterologous (c, f &h) fusion proteins. (B) Antigens including HeLa lysates (lanes 1), C. trachomatis-infected HeLa (Ct-HeLa) lysates (lanes 2) and fusion proteins GST-CT311 (lanes 3) and GST-CPAF (lanes 4) were resolved in SDS-polyacrylamide gels and blotted onto nitrocellulose membrane for detection with antibodies against CT311 (mAb 7C10, panels a-c), CPAF (mAb 100a, d-f), MOMP (mAb MC22, g) and human HSP70 (mAb W27, h) with (b, c, e & f) or without (a, d, g & h) absorption with corresponding (b & f) or heterologous (c & e) fusion proteins. Note that the mouse anti-CT311 mAb only reacted with the endogenous CT311 and the GST-CT311 without cross reacting with any other GST fusion proteins or any other proteins from the C. trachomatis-infected cells. CPAFc represents the C-terminal fragment of CPAF generated as a result of CPAF processing occurring in chlamydia-infected cells. The epitope recognized by mAb 100a is located in the C-terminal fragment. In addition, the anti-CT311 mAb detected fast migrating protein bands besides the full length of CT311 or GST-CT311, suggest that CT311 may be processed. (C) The quality of fusion proteins used in the Western blot assay was monitored in a Coomassie blue staining gel. Full length GST-CT311 and GST-CPAF were detected although degradation fragments from GST-CT311 were also observed. All Fig. 2 experiments were repeated 3 times.