Figure 4.
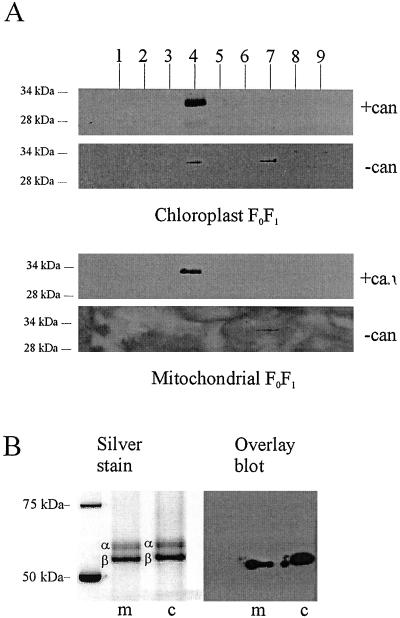
14-3-3B protein copurifies with FoF1 and interacts with the β-subunit. (A) Partially purified FoF1 [isolated in the presence or absence of cantharidin (can)] was further purified by gel filtration; proteins within each fraction (1 to 9) were separated by SDS/PAGE and blotted onto nitrocellulose. Blots were probed with antibody specific for 14-3-3B. In the presence of cantharidin, a single 14-3-3 band was seen in fraction 4, which is the fraction containing the peak of FoF1 (as determined by hydrolytic activity and silver staining; see B). When FoF1 was isolated in the absence of cantharidin, the amount of 14-3-3 in fraction 4 was strongly reduced (CFoF1) or zero (MFoF1). A small amount of 14-3-3 was found in fraction 7, which represents proteins with an apparent molecular mass between 45 and 70 kDa (the mass of uncomplexed 14-3-3 dimer). (B) Fraction 4 of the gel filtration experiments shown above was run on SDS/PAGE and silver stained to show the position of the α- and β-subunits. A second gel was blotted and probed with biotinylated recombinant 14-3-3B. The bound 14-3-3B was detected with streptavidin-HRP. The overlay assay yields a band of 55 kDa in the case of mitochondrial extract, and 57 kDa in the case of the chloroplast. The comparison with the silver-stained gel shows that these molecular masses correspond to the estimated molecular mass of chloroplast and mitochondrial ATP synthase β-subunit, respectively.