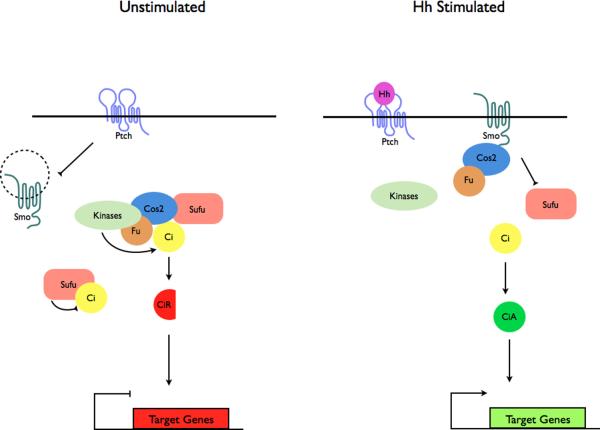
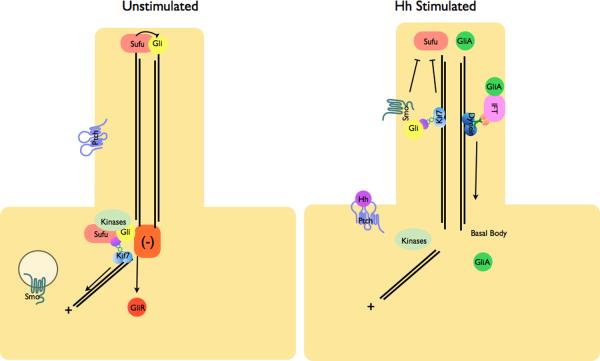
Figure 3. Localization of Hh pathway complexes in Drosophila and mammals.
a | Modulation of protein complex structure and localization in Drosophila by Hh. In the absence of ligand, Ptch prevents translocation of Smo to the plasma membrane. A microtubule-associated complex including Cos2, Fu, Sufu and Ci recruits kinases including PKA, CK1, GSK3β that promote the processing of Ci into its repressor form (CiR)130–133. Sufu may also associate with full length Ci to prevent its translocation to the nucleus130. Upon activation of the pathway, Smo moves to the plasma membrane, and the Fu-Cos2 complex associates with the C-terminal tail of Smo resulting in the release of Ci. Pathway activation also inactivates the negative regulator130–133. b | In vertebrate Hh signaling, signal transduction takes place within cilia, but the behavior of protein complexes may parallel that in Drosophila. In the absence of ligand, Ptch localizes to the cilium, and is thought to block the entry of Smo into cilia35. Kif7 (the Cos2 homologue) localizes to the base of the cilium55 where it may form a complex Gli proteins and other pathway components. Kif7 at the cilium base prevents Gli enrichment within the cilium and promotes processing of GliR. Upon activation of the pathway, Smo moves to the ciliary membrane and Kif7 translocates into the cilium thereby promoting Gli2 accumulation at the cilium tip40, 55. Kif7 at the cilia tip may also block the function of Sufu. Activated Gli is transported out of the cilium by Dynein and IFT particles.