FIGURE 2.
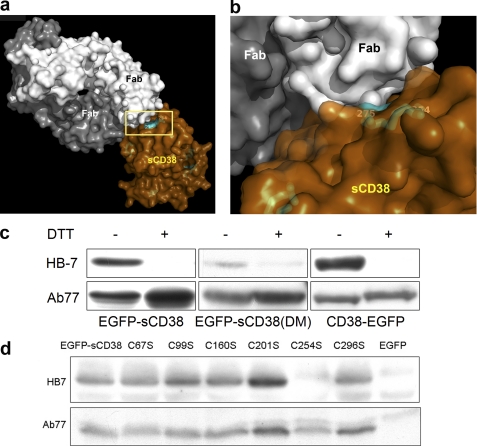
Engineered CD38 possesses intact disulfides. a, crystal structure of CD38 complexed with antibody HB7. The catalytic domain of CD38 (brown) was produced in yeast and complexed with the Fab fragments of HB7 (white and gray). b, close-up view of HB7 binding specifically to the surface region of CD38 defined by the disulfide linkage Cys-254–Cys-275 (cyan). c, specific recognition of the nonreduced form of the engineered CD38 by HB7. Extracts from HEK293 cells expressing EGFP-sCD38, EGFP-sCD38(DM), or wtCD38-EGFP were analyzed by immunoblotting using either monoclonal antibody HB7 or polyclonal antibody Ab77 against CD38. SDS-PAGE was performed either under reducing (+DTT) or nonreducing (−DTT) conditions. HB7 recognizes wild-type or engineered CD38 only under nonreducing conditions. d, extracts from HEK293 cells expressing EGFP-sCD38, its cysteine mutants (C67S, C99S, C160S, C201S, C254S, or C296S), or only EGFP as a negative control were analyzed by immunoblotting using either monoclonal antibody HB7 or polyclonal antibody Ab77 against CD38 under nonreducing conditions (without DTT). HB7 recognizes EGFP-sCD38 and all its cysteine mutants except C254S.