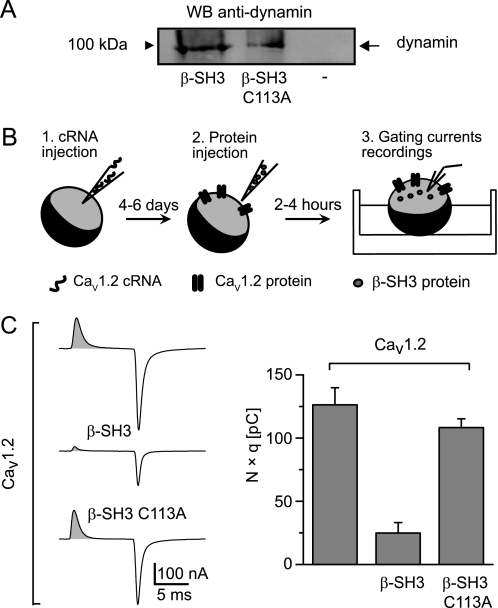
FIGURE 2.
β-SH3 C113A dimerization-deficient mutant preserves its association with dynamin but loses the endocytic capability. A, Western blot of a pull-down assay using His-tagged wild-type and C113A β-SH3 as bait. Lysates from cells expressing dynamin were incubated with either β-SH3 (lane 1) or β-SH3 C113A (lane 2) precoupled to cobalt beads or with cobalt beads alone (lane 3). Bound proteins were detected with anti-dynamin antibody. B, a schematic describing the experimental protocol for CMBI assay. 1, Xenopus oocytes are first injected with CaVα1-encoding cRNA; 2, after a few days, when the channels are already expressed at the plasma membrane, oocytes are reinjected with the test protein; and 3, a few hours later, gating currents are recorded. C, representative gating currents recordings and CMBI assay results from Xenopus oocytes expressing CaV1.2 alone and following injection of either β-SH3 or β-SH3 C113A. The shaded area under the gating current represents the total amount of charges moved during the voltage step and equals the number of channels (N) times the number of charges displaced per channel (q). The bar graph shows N × q values from oocytes expressing the indicated channel-protein combinations; CaV1.2 alone (120 ± 13 pC, n = 14), CaV1.2+β-SH3 (25 ± 8 pC, n = 12), and CaV1.2+β-SH3 C113A (108 ± 7 pC, n = 17). Average N × q values of CaV1.2+β-SH3 oocytes were significantly smaller that for CaV1.2 alone (p < 2.0 × 10−6) or CaV1.2+β-SH3 C113A (p < 1.3 × 10−8) according to a two-tailed t test.