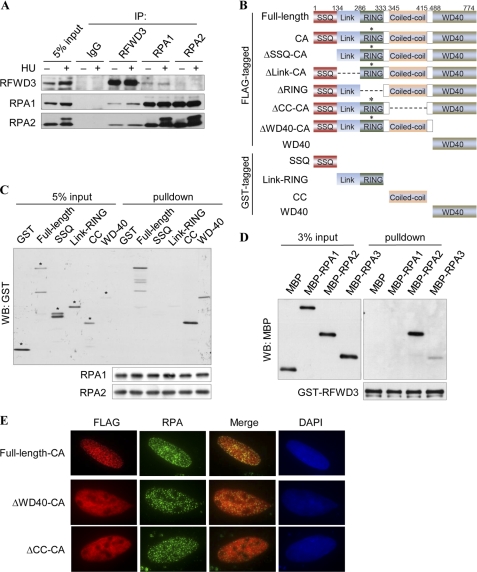
FIGURE 2.
RFWD3 interacts with RPA in vivo and in vitro. A, co-immunoprecipitation of endogenous RFWD3 and RPA was performed with nuclear extracts prepared from untreated or HU-treated HeLa cell. B, domain structure and schematics of the RFWD3 fragments are shown. C315A mutation in the RING domain abolished E3 activity. C, recombinant GST-RFWD3 (full-length or domain-deletion mutants) were incubated with SFB-RPA bound to the FLAG M2 beads. Bound proteins were eluted with Laemmli buffer and immunoblotted (WB) with the indicated antibodies. Asterisks (*) denote the intended bands. D, recombinant GST-RFWD3 bound to Glutathione-Sepharose beads was incubated with MBP-tagged RPA1, RPA2, or RPA3. Bound proteins were immunoblotted with the MBP antibody. E, U2OS cells were transfected with plasmids encoding FLAG-tagged RFWD3 or its deletion mutants and treated with HU. Cells were fixed and immunostained with FLAG and RPA antibodies.