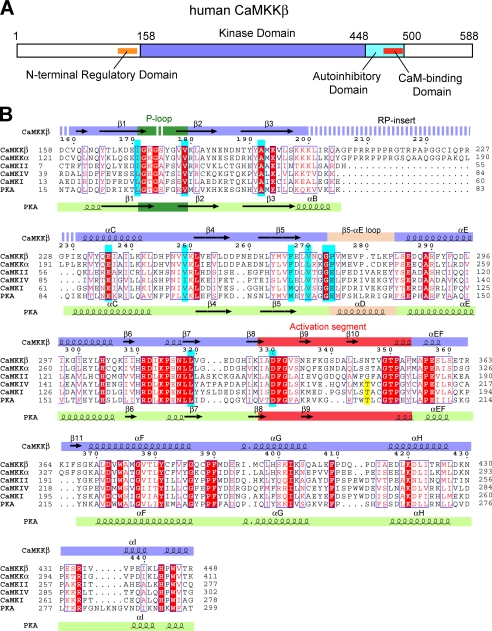
FIGURE 1.
Primary structure of CaMKKβ. A, domain organization of human CaMKKβ. B, sequence alignment of the KDs of human CaMKKβ, human CaMKKα, human CaMKI, human CaMKIV, and mouse PKA. The STO-609-interacting residues are colored cyan, and the activation loop phosphorylation sites are yellow. The secondary structural elements of CaMKKβ and PKA are indicated above and below the sequences, respectively. The glycine-rich P-loop is colored green, the activation segment is red, and the β5-αE loop (CaMKKβ) and the helix αD (PKA) are wheat. Disordered regions are indicated as dashed lines. This figure was generated using the program ESPript (49).