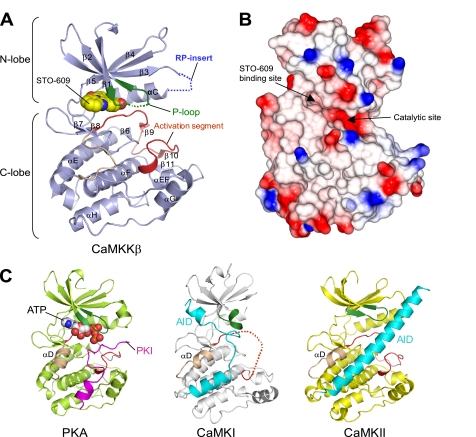
FIGURE 2.
Structure of the CaMKKβ·STO-609 complex. A, ribbon representation of the CaMKKβ KD structure. The P-loop is colored green, the activation segment is red, and the β5-αE loop is wheat. The STO-609 is shown in a sphere representation. Disordered regions are indicated as dashed lines. B, the electrostatic surface of the CaMKKβ KD (red, negative charge; blue, positive charge), shown in the same orientation as in A. The locations of the STO-609-binding site and the catalytic site are indicated. This figure was generated using the program CCP4 MG (50). C, structure comparison with other protein kinases. Structures of the PKA KD complexed with PKA inhibitor (PKI) (left), the CaMKI KD-AID (middle), and the CaMKII KD-AID (right), shown in the same orientation as that of CaMKKβ (A). In these KD structures, the P-loop is colored green, the activation segment is red, and the helix αD is wheat. The PKI bound to PKA is colored magenta, and the AIDs of CaMKI and CaMKII are cyan. The ATP bound to PKA is shown in a sphere representation.