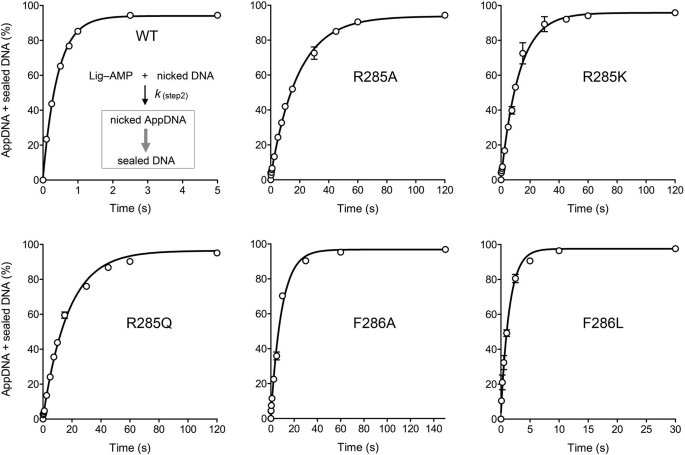
FIGURE 3.
Kinetics of single-turnover DNA adenylylation and nick sealing by pre-adenylylated ChVLig-AMP. A Kintek RQF3 rapid chemical quench apparatus was used to assay the reaction of nicked DNA with a 10-fold molar excess of preformed ChVLig-AMP at 22 °C in the absence of added ATP, in the range of 0.1–5-s reaction times. Where applicable, longer reaction times were assayed manually. The reaction mixtures contained 50 mm Tris-HCl (pH 7.5), 5 mm DTT, 10 mm MgCl2, 500 nm wild-type or mutant ChVLig-AMP, and 50 nm 5′ 32P-nick-labeled DNA substrate. The rapid kinetic measurements were initiated by mixing two buffer solutions (20 μl each of 50 mm Tris-HCl, pH 7.5, 5 mm DTT, 10 mm MgCl2) containing 100 nm nicked DNA substrate and 1 μm ChVLig-AMP, respectively. The reactions were quenched by rapid mixing with 110 μl of 90% formamide, 50 mm EDTA. The products were analyzed by denaturing PAGE, and the distributions of 32P-labeled DNAs (as sealed 36-mer DNA product, 18-mer AppDNA intermediate, and residual 18-mer pDNA substrate) were quantified by scanning the gels. The rate of step 2 catalysis (DNA adenylylation) was gauged by plotting the sum of AppDNA plus sealed DNA (see the reaction scheme in the top left panel) as a function of reaction time for the wild-type and mutant ChVLig preparations. Each datum in the graphs is the average of three separate kinetic experiments ±S.E. Nonlinear regression curve fitting of the data to a single exponential was performed in Prism; the calculated step 2 rate constants are compiled in Table 3.