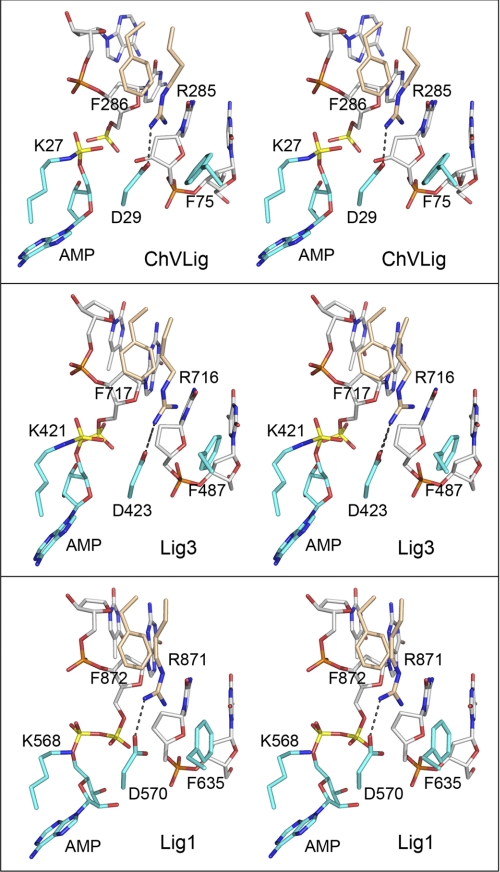
FIGURE 5.
The ChVLig Arg-285-Phe-286, Phe-75, and Asp-29 interaction network at the nick is conserved in human DNA ligases. Aligned stereo images of DNA-bound ChVLig (from 2Q2T; top panel), human Lig3 (from 3L2P; middle panel), and human Lig1 (from 1X9N; bottom panel) highlighting the terminal dinucleotides of the nicked strands (a 5′-PO4/3′-OH in ChVLig, a 5′-PO4/3′-H in Lig3, or an AppDNA/3′-H in Lig1), the motif I lysine (lysyl-AMP in ChVLig and Lig3; lysine in Lig1), the motif I aspartate, the OB domain Arg-Phe dipeptide, and a phenylalanine of the NTase domain are shown. NTase residues are rendered with cyan carbons; OB residues are rendered with beige carbons, and DNA is rendered with gray carbons. The adenylate and nick 5′-phosphorus atoms are colored yellow. The Arg-Asp salt bridges are denoted by dashed lines.