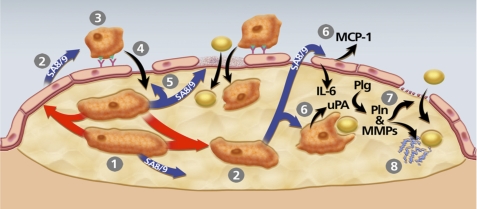
FIGURE 9.
Model of pathways through which macrophage uPA expression could accelerate atherosclerosis. The model is constructed based on data from the present study and others showing that macrophage overexpression of uPA promotes early lesion lipid accumulation as well as later lesion macrophage accumulation, increases macrophage migration, enhances vascular matrix metalloproteinase (MMP) activity, accelerates lesion progression, and stimulates macrophage S100A8/A9 expression (13–16). 1, uPA-expressing macrophages begin to accumulate within an early lesion. 2, These macrophages secrete S100A8/A9 (SA8/9) themselves and can also up-regulate S100A8/A9 expression in other plaque cells (red arrows; see also Fig. 8) including macrophages and potentially in endothelial cells and smooth muscle cells, both of which can express S100A8/A9 (62, 63). 3, S100A8/A9 secreted by lesion cells increases monocyte CD11b expression and adhesion to endothelial ICAM-1 (57, 58). 4, as monocytes differentiate to macrophages, they up-regulate uPA expression, which enhances their migration into the lesion (14). 5, S100A8/A9 stimulation of endothelium also loosens tight junctions and promotes endothelial apoptosis (60, 64), effects that facilitate entry of both monocyte/macrophages and lipids to the artery wall. 6, lesion-derived S100A8/A9 binds to RAGE on endothelial cells and TLR4 on macrophages and smooth muscle cells, up-regulating expression of atherogenic cytokines MCP-1 and IL-6 in endothelium, and increasing lipid uptake by cells within the lesion (63, 65). 7, increased endothelial basement membrane proteolysis by uPA/Plg-activated matrix metalloproteinases (MMPs) (16) also increases artery wall permeability. 8, proteolysis of matrix protein within lesions exposes neoepitopes with apolipoprotein-binding activity, increasing lesion lipid retention.