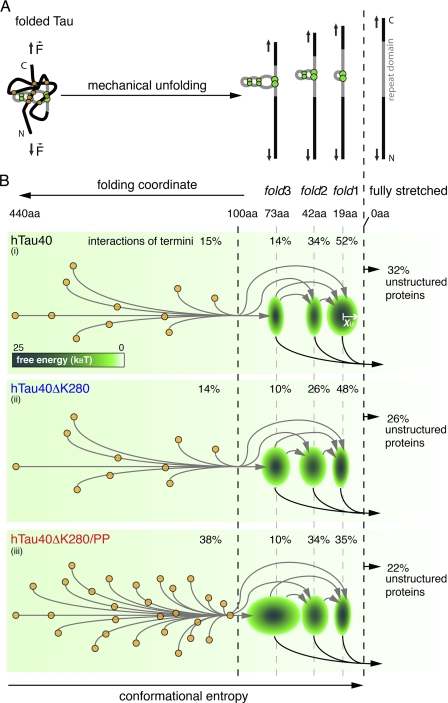
FIGURE 5.
Model of the unfolding energy landscape of full-length human Tau. A, scheme showing the main unfolding intermediates when stretching full-length Tau protein. Tau conformations (left) stabilized by interactions of the termini (orange circles) and the repeat domain (green circles) are forced into fully stretched conformations (right). Thereby, Tau unfolds stepwise taking the main unfolding intermediates stabilized by fold3, fold2, and fold1. B, schematic energy landscapes for the stretching of hTau40 (i), hTau40ΔK280 (ii), and hTau40ΔK280/PP (iii). The three main intramolecular interactions p1, p2, and p3 (green ellipses) establish energy wells in the unfolding landscape of Tau. The widths (x axis) of the three ellipses at 19 (p1), 42 (p2), and 73 aa (p3) estimate the widths xu of the energy wells stabilizing fold1, fold2, and fold3. The depth of every energy well, ΔG‡, is indicated by the color-coded scale bar. Different Tau conformations show different combinations of interactions in the termini and the repeat domain and stepwise unfold through various unfolding pathways (arrows in B, i, ii, and iii). Weak interactions of the termini break before the three abundant folds, fold1, fold2, and fold3, in the repeat domain. The larger number of unspecific, long peptide folds and the low stiffness of the fold3 interaction in hTau40ΔK280/PP (iii) may protect this Tau mutant from establishing aggregation conformations. In contrast, the ∼19- (fold1) and ∼42-aa (fold2) interactions are strengthened in hTau40ΔK280 (ii) and may thus be important for the aggregation of Tau.