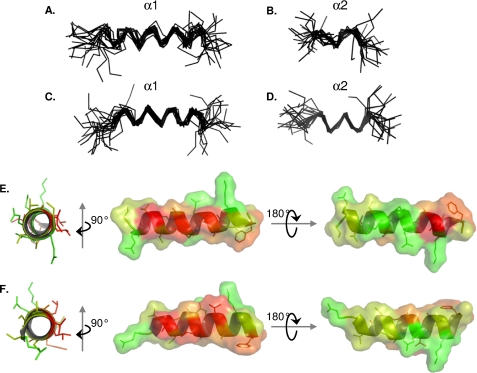
FIGURE 8.
NMR structure model of α1 and α2 helices of NS5A-D3. Shown are superimpositions of the backbone atoms (N, Cα, and CO atoms) of the 20 final structures of NS5A-D3 from JFH-1 HCV strain (A and B) and of NS5A-D3 from Con1 HCV strain (C and D). Superimpositions of the final NS5A-D3 structures were done over the backbone atoms of either the α1 helix (A and C) or the α2 helix (B and D). The amphipathic character of the α1 helix from NS5A-D3 (JFH-1) and from NS5A-D3 (Con1) is shown in E and F, respectively. The average α1 helix structures from both HCV strains are represented as a schematic and colored according to the Kite and Doolittle hydrophobicity scale (80) (−4.5 (green) to +4.5 (red)). The molecular surfaces are shown in the right panels and are colored using the same criteria as described above.