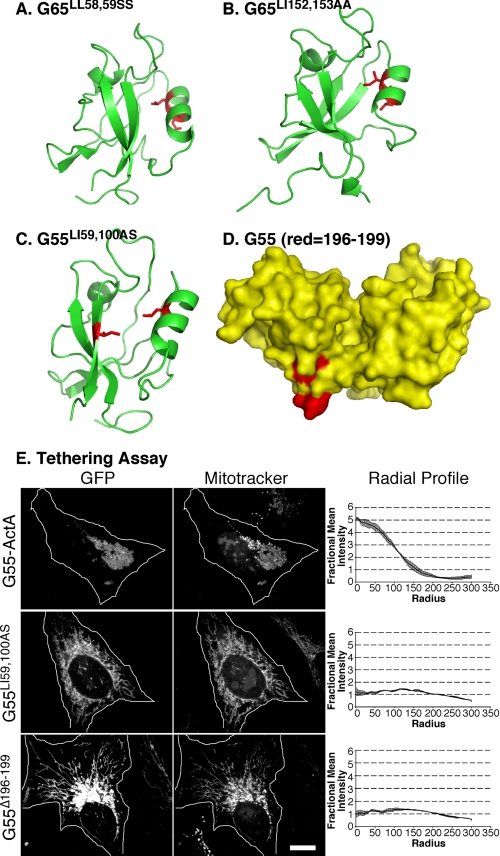
FIGURE 3.
Binding groove and surface mutations that block PDZ function. A and B, representations of GRASP65 modeled after the crystal structure of GRASP55 showing binding groove residues (red) that blocked tethering (A) and Golgi localization (B). C, representation of the pocket residues (shown in red) introduced into GRASP55 PDZ1. D, a surface representation of GRASP55 highlighting the internal ligand residues on PDZ2 that we targeted for deletion (shown in red). E, HeLa cells expressing the wild-type construct (G55-GFP-ActA), the pocket mutant construct (G55LI59,100AS), or the deleted internal ligand construct (G55Δ196–199) were analyzed after a 24-h transfection. Cells were imaged using GFP fluorescence to identify transfected proteins and MitoTrackerTM to identify mitochondrial membranes. Quantification was by radial profile analysis (2, 7, 8), which reports averages corresponding to the fraction of total fluorescence present in each concentric circle drawn from the centroid.