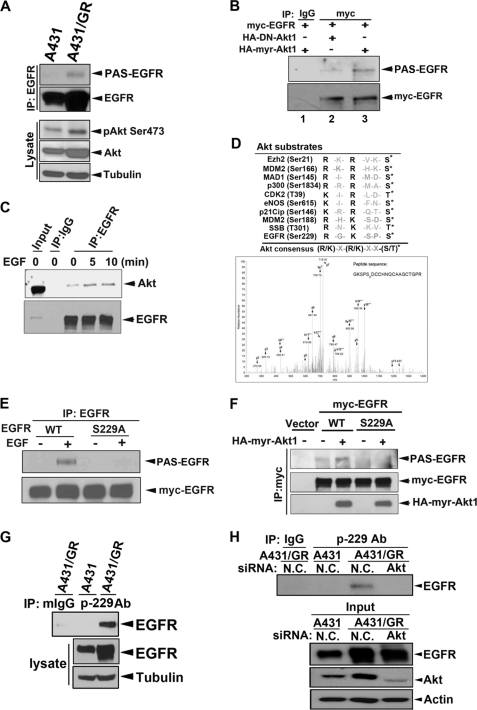
FIGURE 2.
Akt phosphorylates EGFR at Ser-229. A, whole cell lysates prepared from cells were subjected to IP/WB analysis by using indicated antibodies. PAS, anti-phospho-Akt-substrate antibody. B, HEK293 cells were transfected with the indicated constructs and then subjected to IP/WB analysis. C, MDA-MB-468 cells were treated with EGF for indicated times, and IP/WB analysis was performed to assess the physical interaction between Akt and EGFR. D, in vivo EGFR Ser-229 phosphorylation was detected in anti-EGFR immunoprecipitates from EGF-treated MDA-MB-468 cells by mass spectrometry. E and F, substitution of Ser-229 to Ala abolished EGF- or Akt-induced EGFR phosphorylation detected by anti-PAS antibody in anti-EGFR (E) or anti-myc (F) immunoprecipitates from transfected HEK293 cells. G, endogenous EGFR Ser-229 phosphorylation was detected in A431/GR cells by using anti-phospho-EGFR Ser-229 for IP and anti-EGFR antibody for subsequent WB. H, Akt expression in A431/GR cells was deprived by Akt siRNA. Then endogenous EGFR Ser-229 phosphorylation was detected by using anti-phospho-EGFR Ser-229 for IP and anti-EGFR antibody for subsequent WB. N.C., non-specific control.