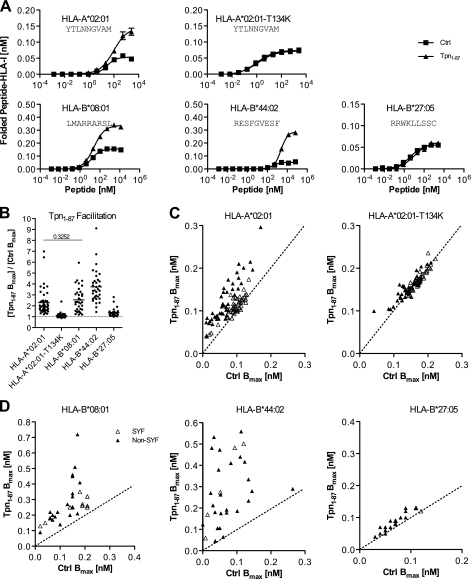
FIGURE 1.
Tpn1–87 facilitates folding of peptide-HLA-I complexes according to peptide and HLA-I identity. A, folding of peptide-HLA-I complexes in single peptide dose-response experiments. Fixed concentrations of β2m and HLA-I HCs were mixed with titrated concentrations of peptide in the presence (▴) or absence (■) of Tpn1–87. The mixtures were incubated at 18 °C for 48 h, and folded peptide-HLA-I complexes were detected by the HLA-I conformation-specific W6/32 monoclonal antibody in a homogenous assay (36). B, a study of Tpn1–87 facilitation based on the maximum amount of folded peptide-HLA-I complexes, Bmax. Peptide dose-response curves were made by offering each peptide in different concentrations to the folding reaction. The saturation plateaus were calculated as Bmax from the curves. Binding curves were made in the presence (Tpn1–87 Bmax) and absence (Ctrl Bmax) of Tpn1–87 with SYFPEITHI (△) and non-SYFPEITHI (▴) peptides. B, the degree of Tpn1–87 facilitation for each of the tested HLA-I molecules is shown. C, the Bmax values with and without Tpn1–87 for the binding of each of the tested peptides to HLA-A*02:01 and HLA-A*02:01-T134K are plotted. D, the Bmax values for the binding of peptides specific for HLA-B*44:02, -B*08:01, and -B*27:05 with and without Tpn1–87 are plotted. All of the experiments were done in quadruplicate, and standard deviations for each folding reaction were calculated and visualized in the graphs. A Student's t test was applied to determine whether the means were significantly different. All of the means were significantly different (p < 0.001), except for the one with the p value shown in the graph.