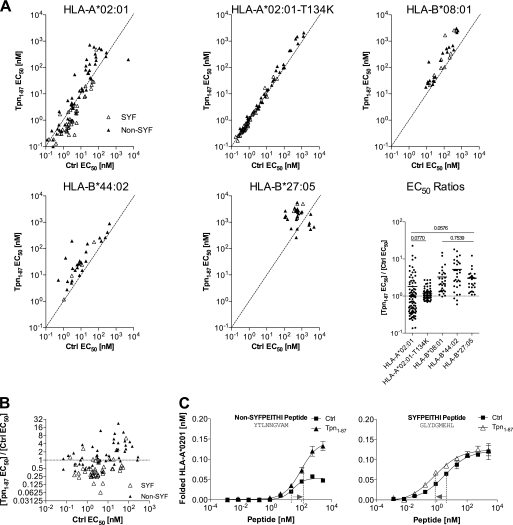
FIGURE 2.
Tpn1–87 influences the peptide binding affinity to HLA-I. A, Tpn1–87 influences the peptide affinity to HLA-I. Multiple binding curves were made, and the peptide concentration resulting in half-saturation was calculated as EC50. Binding curves were made in the presence (Tpn1–87 EC50) and absence (Ctrl EC50) of Tpn1–87. 44 SYFPEITHI (△) and 44 non-SYFPEITHI (▴) peptides were tested on HLA-A*02:01. The same peptides were tested on HLA-A*02:01-T134K. Other peptide panels were tested on HLA-B*08:01, HLA-B*44:02, and HLA-B*27:05. The EC50 ratios (Tpn1–87 EC50/Ctrl EC50) are shown in the EC50 ratios graph. A Student's t test was used to determine whether the means were significantly different (p < 0.05) between the HLA-I molecules. The p values are shown in cases where no significant differences were found (for a complete list see supplemental Table S2). B, the EC50 ratios on HLA-A*02:01 were plotted against Ctrl EC50 and grouped in non-SYFPEITHI and SYFPEITHI peptide groups. C, peptide dose-response curves representing the non-SYFPEITHI and SYFPEITHI peptide-HLA-A*02:01 complexes were analyzed for shifts in EC50. For the non-SYFPEITHI peptide, the EC50 values increased in the presence of Tpn1–87 corresponding to a decrease in affinity. For the SYFPEITHI peptide, the EC50 values decreased in the presence of Tpn1–87, corresponding to an increase in affinity.