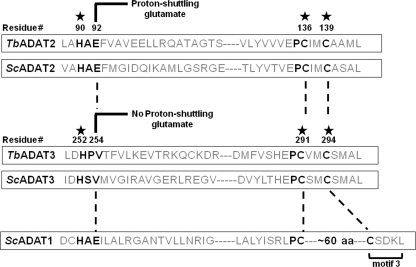
FIGURE 1.
Evolutionarily conserved residues in the active sites of TbADAT2 and TbADAT3. The figure shows a schematic highlighting conserved amino acids involved in Zn2+ coordination in TbADAT2, ScADAT2, TbADAT3, and ScADAT3 (denoted by a star). The key glutamate required for catalysis is as indicated. This glutamate is involved in shuttling a proton from an activated water molecule into the leaving amino group, which is released as ammonia during the reaction. The glutamate has been naturally replaced during evolution by a valine in Sc and TbADAT3. The numbers indicate amino acid positions in each sequence relative to the first methionine in T. brucei. Key deaminase residues are highlighted with black letters. motif 3 refers to a motif characteristic of adenosine deaminase (ADAT1 for example), where the second zinc-coordinating cysteine is separated from the first by an ∼60-amino acid spacer. This motif is never found in cytidine deaminases.