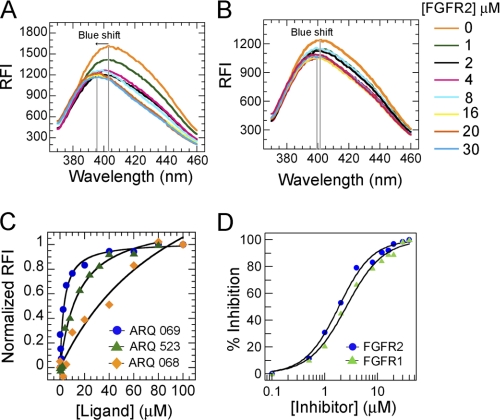
FIGURE 2.
ARQ 069 binds to a hydrophobic region and enantioselectively inhibits FGFR activity. A, intrinsic fluorescence emission change of ARQ 069 upon binding to inactive FGFR2. The fluorescence emission spectra of ARQ 069 at a concentration of 1 μm were measured at an excitation wavelength of 332 nm in the presence of increasing concentrations of FGFR2 protein. Interaction of FGFR2 with ARQ 069 is accompanied by a shift in the emission maximum followed by a decrease in fluorescence intensity. B, ARQ 068 did not show a significant change in the emission maximum or emission intensity in the presence of increasing FGFR2 concentrations. The concentrations of FGFR2 used to determine the binding of both ligands are shown. C, the affinity of ARQ 523, ARQ 069, and ARQ 068 to FGFR2 was measured by following the intrinsic tryptophan quench and recording the fluorescence emission at 345 nm at various concentrations of ligand. The FGFR2 concentration was held constant at 0.5 μm. D, inhibition of FGFR1 and FGFR2 autophosphorylation by ARQ 069. The autophosphorylation reaction was followed by continuously monitoring the decrease in absorbance at 340 nm. Formation of ADP was coupled with the oxidation of NADH. The kinase domains of FGFR2 or FGFR1 (1 μm) were incubated at increasing concentrations of ARQ 069 (0 to 40 μm), the reaction was initiated with the addition of 1 mm ATP. IC50 values were determined by fitting the rate of autophosphorylation inhibition at various concentrations of ARQ 069.