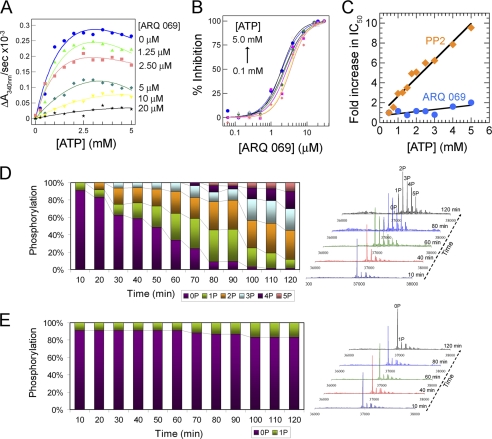
FIGURE 3.
Inhibition of FGFR2 autophosphorylation by ARQ 069 is not dependent on ATP concentration. A, FGFR2 (1 μm) was preincubated at the indicated concentrations of ARQ 069. Autophosphorylation reaction rates were evaluated across a wide range of ATP concentrations. The reaction was followed by a continuous spectrometric assay measuring ADP formation coupled to the NADH oxidation reaction, which monitors a decrease in absorbance at 340 nm. Activation of FGFR2 increased hyperbolically with increases in ATP concentrations and the rate of autoactivation was dramatically reduced upon preincubation with ARQ 069. Above a 20 μm concentration of ARQ 069, the inhibition of FGFR2 activation does not change significantly up to the highest ATP concentration of 5 mm. B, dose-response curves were derived from the same experiments. ARQ 069 retained its ability to inhibit FGFR2 autophosphorylation at all concentrations of ATP as indicated by similar IC50 values. C, in a similar experiment, the non-ATP competitive inhibitor of Src, PP2, showed ATP competitive inhibition kinetics with FGFR2 kinase as reflected by a linear dependence of IC50 values as a function of ATP. ARQ 069 behaves kinetically as non-ATP competitive when tested in a similar ATP concentration range as used for PP2. D, FGFR2 (2 μm) autophosphorylation was also monitored by ESI-MS as a function of time at 5 mm ATP concentration. The right panel shows the deconvolted MS spectra at representative time points. E, in a similar autophosphorylation experiment at 5 mm ATP concentration, FGFR2 preincubated with 20 μm ARQ 069 showed strong phosphorylation inhibition as reflected by the weak autophosphorylation levels. Together these results support an ATP-independent mechanism of inhibition of FGFR2. Details of the MS experiments for D and E can be found under “supplemental Methods”.