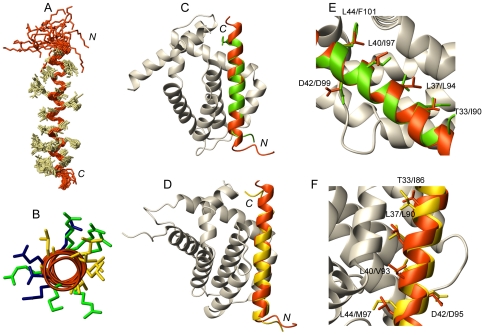
Figure 6. Three-dimensional structure of the cytosolic fragment of Hrk in TFE (residues 22–53) compared to BH3-peptides in heterodimer structures.
(A) Backbone superposition of the 20 conformers with lowest energy. Side chains are shown in ivory. N and C termini are indicated. (B) Top view of the helix formed by Hrk-22_53 showing in yellow the clustering of hydrophobic residues Leu and Ala flanked by polar residues Lys, Arg (in green) and Gln, Glu and Asp (in blue). (C, D) Superposition of Hrk-22_53 (red) and the helix formed by a peptide comprising the BH3 domain of the BH3-only protein Bid (green) in complex with prosurvival Mcl-1 (gray, PDB ID 2KBW) (C) and the BH3 domain of the BH3-only protein Bim (yellow) in complex with prosurvival Bcl-xL (gray, PDB ID 3FDL) (D). (E, F) Side chain position of the four conserved hydrophobic residues anchoring the helix to the prosurvival protein groove and highly conserved Asp in Hrk-22_53 (red), Bid-BH3-peptide (green, PDB ID 2KBW) (E), Bim-BH3 peptide (yellow, PDB ID 3FDL) (F). Residues are labeled.