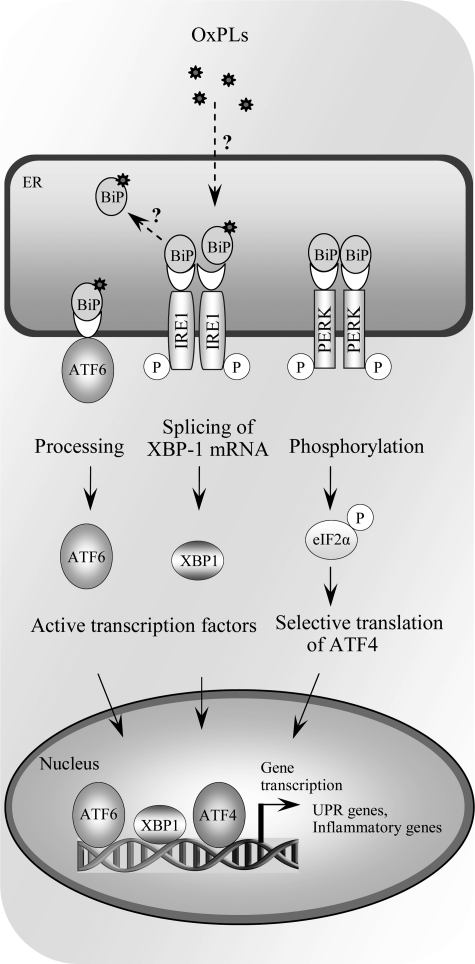
FIG. 9.
Activation of unfolded protein response (UPR) by OxPLs. Multiple stress conditions leading to impaired processing of proteins in endoplasmic reticulum activate adaptive reaction called UPR, which is mediated via three branches, all initiated by binding of unfolded proteins to BIP/GRP78, thus leading to dissociation from, and de-repression of, ATF6, IRE1, and PERK. Dissociation of BIP/GRP78 initiates processing of ATF6 protein, splicing of XBP-1 mRNA by IRE1, and phosphorylation of eIF2α, resulting in selective translation of ATF4. OxPAPC and other classes of OxPLs were shown to activate all three arms of the UPR, leading to formation of transcriptionally active ATF6, XBP1, and ATF4, as well as induction of their target genes. However, the mechanism of activation is currently unknown. In particular, it is not clear whether similarly to unfolded proteins OxPLs directly bind to BiP/GRP78 and reverse repression of ATF6, IRE1, and PERK.