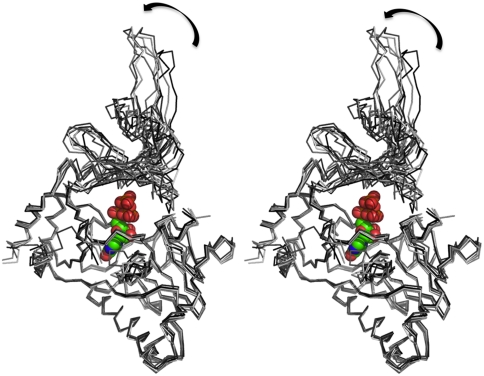
Fig. 3.
Structural variations among the hGTase molecules. A stereoview showing the superposition of the seven hGTase molecules in the asymmetric unit. The large variations in the positioning of the OB-domain with respect to the nucleotide transferase domain reveals the mode of interdomain movements that is essential for the activity of the enzyme. In the DNA ligase domain, the GTP-binding pocket is highly superimposable; however, the distant structural motif β6–β7 hairpin shows structural variation. The GTP molecule is positioned based on structural superposition of the Chlorella PBCV-1 virus GTase (PDB ID code 1CKN-A) on hGTase.