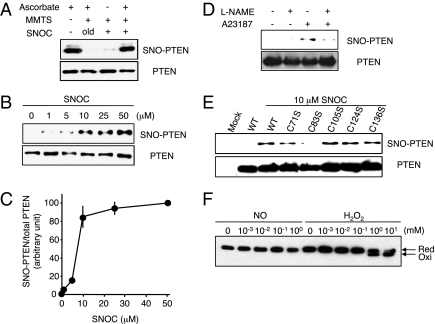
Fig. 1.
S-nitrosylation of PTEN in vitro and in vivo. (A, Upper) Cell lysates from HEK293T cells were incubated with 50 μM SNOC or control solution for 20 min, followed by assay for SNO-PTEN using the biotin-switch assay. Control samples were subjected to decayed (old) SNOC. (Lower) Total PTEN in cell lysates was detected by Western blot. (B, Upper) HEK293T cells were exposed to varying concentrations of SNOC for 20 min, followed by assay for SNO-PTEN. (Lower) Total PTEN. (C) Biotin-switch assay and Western analysis were quantified by densitometry; the relative ratio of SNO-PTEN to total PTEN was calculated for each sample. Values are means ± SEM, n = 3. (D, Upper) HEK cells expressing nNOS were assayed for endogenous SNO-PTEN. nNOS was activated by Ca2+ ionophore A23187 (5 μM) in the presence or absence of NOS inhibitor (NG-Nitro-l-arginine methyl ester, l-NAME, 1 mM). (Lower) Total PTEN. (E, Upper) S-nitrosylation of Cys-83 in PTEN. HEK cell lysates transduced with WT or C-to-S mutant FLAG-PTEN proteins were exposed to 10 μM SNOC or control for 20 min. SNO-PTEN was detected by biotin-switch assay using anti-FLAG antibody. Mutation of a critical cysteine thiol group in the phosphatase domain of PTEN(C83S) prevented S-nitrosylation by SNOC. (Lower) Total PTEN. (F) Effect of NO on oxidation of PTEN. HEK293 cells were incubated with the indicated concentration of SNOC or H2O2 for 20 min. Cell protein extracts were prepared in lysis buffer (pH 6.8) containing 40 mM N-ethylmaleimide and fractionated by nonreducing SDS/PAGE followed by Western analysis with anti-PTEN antibody.