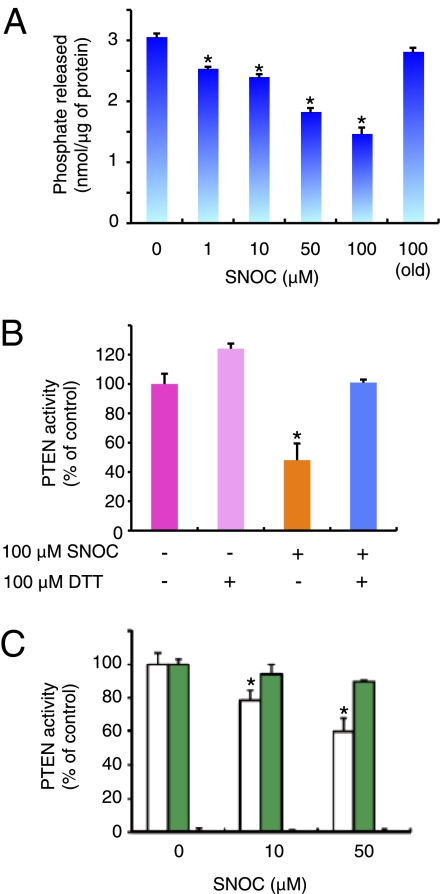
Fig. 2.
S-nitrosylation of PTEN regulates its phosphatase activity. (A and B) Effect of S-nitrosylation on PTEN phosphatase activity. (A) In vitro expressed GST-fused PTEN was incubated with the indicated concentrations of SNOC and evaluated by phosphatase assay. (B) Recombinant PTEN and SNO-PTEN were assayed for lipid phosphatase activity against PI(3,4,5)P3 with or without DTT. Release of phosphate was detected colorimetrically with Biomol green reagent. Values are means ± SEM, n = 5; *P < 0.01 by ANOVA. (C) GST-fused WT-PTEN (white), PTEN(C83S) (green), and dominant-negative PTEN(C124S) (black) were expressed and purified from bacteria, exposed to SNOC, and assayed for phosphatase activity. Values, expressed as percentage of WT in the absence of SNOC, are means ± SEM, n = 5; *P < 0.01 by ANOVA.