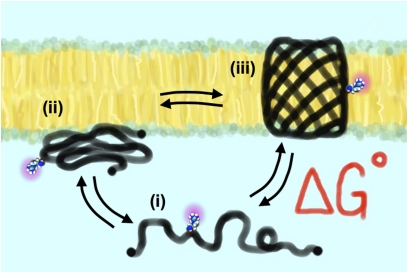
Fig. 1.
Reversible, spontaneous OmpLA folding system. At low pH and in the presence of denaturants, the β-barrel protein is in equilibrium between (i) a soluble unfolded state, (ii) a prefolded intermediate that spontaneously inserts into the lipid bilayer, and (iii) the final folded functional state of the protein. The free energy of the folding, ΔG°, is measured by quantifying the unfolded and folded populations. Moon and Fleming (4) use OmpLA as a host system and measure the change in free energy each residue confers when constrained to the center of the lipid bilayer, providing a whole-protein hydrophobicity energy scale for the 20 natural amino acids, relative to alanine.