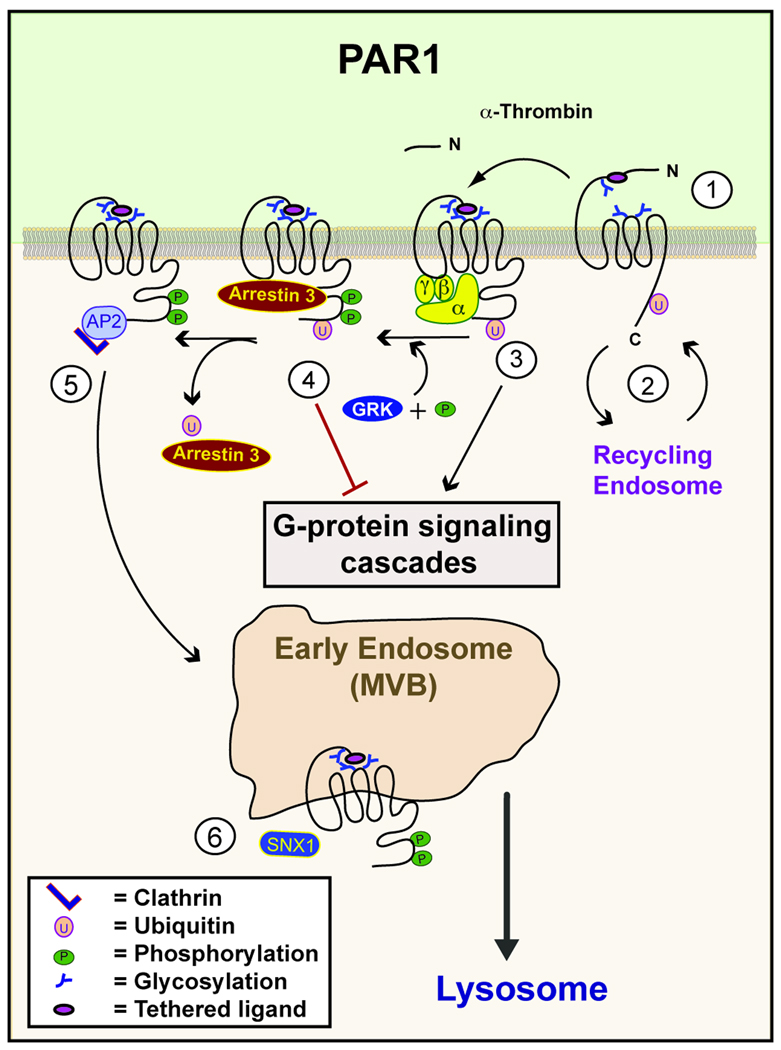
Fig. 1. Regulation of PAR1 signaling by posttranslational modifications.
(1) N-linked glycosylation of PAR1 regulates surface expression and signaling. (2) Basal ubiquitination of PAR1 promotes retention at the cell surface and negatively regulates constitutive recycling. (3) Thrombin binds to and cleaves the N-terminus of PAR1, exposing a new N-terminus that functions as a tethered ligand, which induces conformational changes within the receptor that results in activation of heterotrimeric G proteins. (4) Activation of PAR1 results in rapid phosphorylation mediated by GRKs and the recruitment of arrestins, which promote the dissociation of receptor from G proteins. (5) After activation, PAR1 is internalized through clathrin-coated pits via an AP-2 dependent pathway that occurs independent of arrestins. (6) Once internalized, activated PAR1 is sorted from early endosomes/multivesicular bodies (MVBs) to lysosomes through a poorly understood process mediated by SNX1.