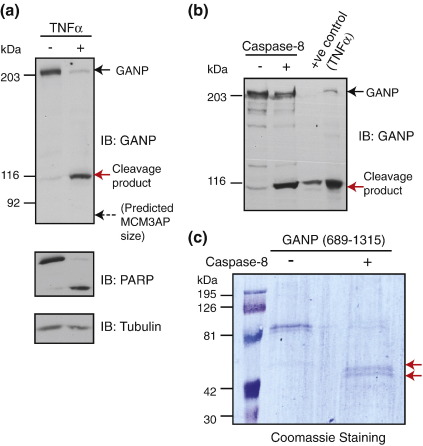
Fig. 3.
GANP is cleaved during apoptosis into a fragment that still contains the MCM3AP sequence. (a) HEK293 cells were treated with TNFa and analysed by Western blotting with antibodies against GANP, PARP (Calbiochem), and tubulin (Abcam). The cleavage product is ∼30 kDa larger than MCM3AP and indicated by a red arrow. Dashed arrow indicates the predicted position of MCM3AP. (b) Recombinant caspase-8 cleaves GANP into a 110-kDa fragment. Structure-bound fraction from HEK293 cells was incubated with recombinant caspase-8 (Calbiochem) for 1 h at 30 °C and analysed by Western blotting with an anti-GANP antibody. (c) Mapping of caspase-8 cleavage site. A fragment of GANP spanning residues 689–1315 was expressed and purified in E. coli, and 100 μg was incubated with recombinant caspase-8 in an in vitro cleavage reaction under identical experimental conditions to the in vivo reaction above. Samples were analysed by SDS-PAGE and Coomassie staining, and N-terminal sequencing was carried out on the bands by the University of Cambridge Department of Biochemistry to determine the identity of the cleavage site.