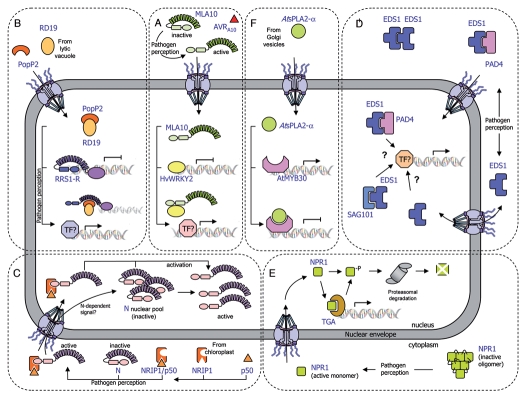
Figure 1.
Schematic representation of the transport of plant immune receptors and regulatory components across the nuclear envelope. (A) The MLA10-mediated immune response is activated by the AVRA10 effector, which promotes nuclear accumulation of CC-NB-LRR resistance protein MLA10. In resting cells, HvWRKY2 acts as a transcriptional repressor of basal defense responses. After pathogen perception, MLA10 binds HvWRKY2 through its CC domain, thereby releasing the negative regulation of the immune response. (B) The PopP2 effector, which triggers the RRS1-R-mediated resistance response, promotes nuclear accumulation of the TIR-NB-LRR-WRKY immune receptor RRS1-R and the vacuolar cysteine protease RD19. In unchallenged cells, RRS1-R is hypothesized to act as a transcriptional repressor of basal defense responses. The RD19/PopP2 protein complex would be recognized by RRS1-R leading to either modification of RRS1-R transcriptional activity or transcriptional activation by additional transcription factors, thereby derepressing defense responses. (C) The tobacco TIR-NB-LRR immune receptor N resides in the cytoplasm and the nucleus of non-infected cells. After pathogen inoculation, the tobacco rhodanase sulfurtransferase NRIP1, which localizes to the stroma of chloroplast in resting cells, is recruited to the cytoplasm of the plant cell by the p50 effector, to form a pre-recognition complex. This NRIP1/p50 complex interacts with the N receptor thanks to NRIP1, which binds to the TIR domain of N, leading to the activation of cytoplasmic N. Once activated, cytoplasmic N either enters the nucleus or sends a signal that activates the N nuclear pool, resulting in the activation of a successful defense response. (D) Basal and TIR-NB-LRR resistance protein-mediated defense responses depend on the regulatory immune complexes formed by the EDS1, PAD4 and SAG101 proteins. EDS1 can exist in a complex with PAD4 in both the cytoplasm and nucleus, and with SAG101 in the nucleus, resulting in the regulation of defense gene transcription. The role of EDS1 homodimers in the cytoplasm is still unknown. Dynamic changes in the binding affinities and concentrations of these complexes modulate nuclear accumulation of defense-related components, which allows the plant to mount an appropriate immune response. (E) The transcriptional regulator NPR1 primarily exists as inactive oligomers in the cytoplasm of non-elicited cells. Pathogen recognition leads to a change in the cell redox potential, resulting in the formation of reduced active NPR1 monomers that are translocated into the nucleus. Within the nucleus, NPR1 associates with TGA transcription factors to promote defense gene expression. Levels of nuclear NPR1 accumulation are controlled by protein degradation through the 26S proteasome. (F) The secreted phospholipase AtsPLA2-α is partially relocalized, via a yet undescribed mechanism, from Golgi vesicles to the nucleus where it interacts with the transcription factor AtMYB30, leading to transcriptional repression of defense responses.