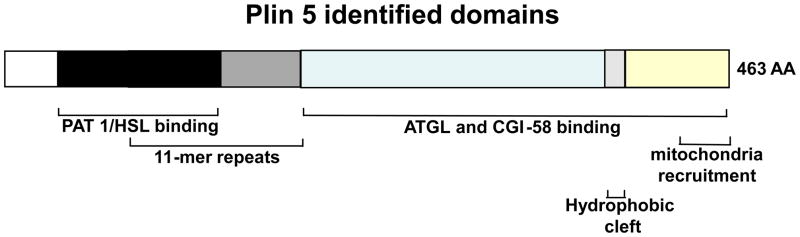
Figure 2. Schematic diagram of the structural features of murine perilipin 5.
Structural domains that are shared by some of the perilipin family of proteins are depicted in shades of black and grey. The N terminus of perilipin 5 contains 100 amino acid (AA) sequences that are highly conserved between perilipin 1, 2, 3 but not 4 (PAT-1 domain, (black)). Overlapping with these sequences are stretches of amino acids containing 11-mer repeat sequences, a common feature for all perilipin proteins (PAT-2 domain, (medium grey)). The C-terminus of perilipin 5 contains a highly conserved sequence of 14 amino acids that folds into a hydrophobic cleft in perilipin 2, 3 and 4 but not in perilipin 1 (light grey). Unique to perilipin 5 are the ATGL and CGI-58 binding domain (blue) between 189 amino acids (AA) and 391 AA as well as a mitochondria-binding domain between 443 AA and 463 AA (yellow).