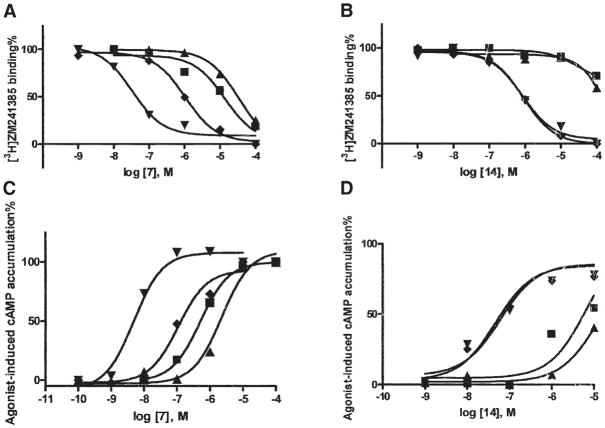
Figure 4. Pharmacological Characterization of Two Neoagonists Indicates Selective Interaction with Neoceptors Derived from the A2AAR.
Binding (A and B) and functional (C and D) effects of two adenosine derivatives, the hydrazide derivative 7 (A) and the N6-(2-methyl-benzyl)-5′-aminoethyluronamide derivative 14 (B) at WT (■) and mutant A2AARs (T88D [▲], N181D [◆], and Q89D [▼]) transiently expressed in COS-7 cells. In the binding experiments, cell membranes (10–20 μg protein) were incubated with the radiolabeled antagonist [3H]ZM241385 (2.0 nM) in duplicate, together with increasing concentrations of the competing nucleoside, in a final volume of 0.4 ml Tris-HCl buffer (50 mM, pH 7.4) at 25°C for 120 min. Results were from a representative experiment performed in duplicate. The Ki values listed in Table 1 were from at least three separate experiments. In the functional experiments, cells expressing WT or mutant receptors were then treated with agonist 7 (C) or 14 (D) in the presence of rolipram (10 μM) and adenosine deaminase (3 units/ml) and incubated at 37°C for 1 hr. cAMP accumulation was determined using a competitive protein binding method [29]. The EC50 values (n = 3) determined for stimulation of cAMP formation were (nM, mean ± SEM): WT, 826 ± 138; T88D, 2970 ± 980; Q89D, 5.1 ± 0.8; N181D, 120 ± 22 for 7; and WT, 5800 ± 1230; T88D, 12,600 ± 3200; Q89D, 58 ± 12; N181D, 52 ± 6 for 14.