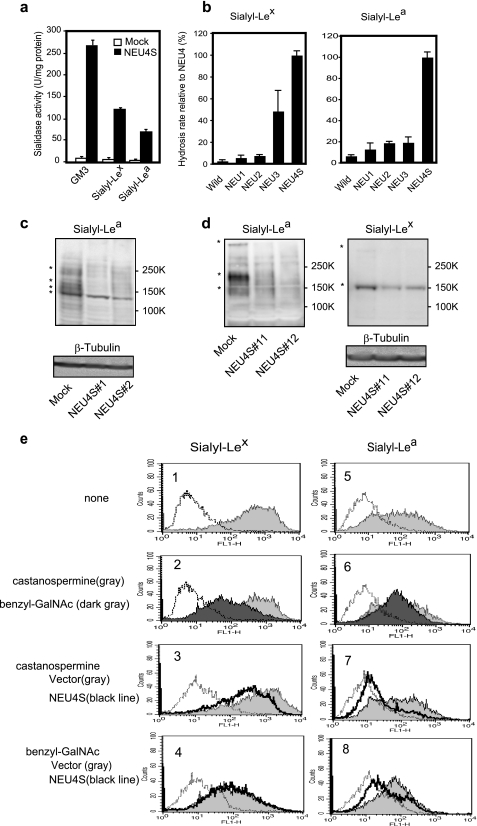
FIGURE 2.
Desialylation of sialyl-Lea and sialyl-Lex by NEU4 and characterization of these glycans. Substrate specificity of NEU4S toward GM3, sialyl-Lex and sialyl-Lea in vitro (a). Different substrate specificity of sialidases NEU1, NEU2, NEU3, and NEU4S toward sialyl-Lex (b, left panel) and sialyl-Lea (b, right). Homogenates from HEK293T cells transfected with the respective sialidase cDNAs were used as enzyme sources, and essentially equal amounts for each sialidase were applied. According to their known substrate specificity, the activity levels were determined in advance with 4MU-NeuAc for NEU1, NEU2, NEU4 and with GM3 for NEU3 and NEU4 as substrates with Triton X-100 (0.1%) or sodium cholate (0.05%). Released sialic acids were estimated by HPLC as described under “Experimental Procedures.” Immunoblot analysis of sialyl-Lea in DLD-1 (c), HT29 (d, left panel) cells, and sialyl-Lex in HT29 cells (d, right). Equal protein loading was confirmed by immunoblots of β-tubulin. Marked decrease in sialyl Lewis antigens is shown with an asterisk. To characterize glycans bearing sialyl Lewis antigens, cells were cultured in the presence of benzyl-GalNAc and castanospermine to inhibit synthesis of O-linked or N-linked glycans, respectively. In both DLD-1 (e, right panel) and HT29 colon cancer cells (e, left panel), treatment with benzyl-GalNAc diminished most of the expression level of both sialyl-Lex (dark gray in 2) and sialyl-Lea (dark gray in 6) at cell surfaces as compared with non-treated cells (gray in 1 and 5), whereas castanospermine (gray in 2 and 6) hardly changed either. The NEU4S-mediated decrease in the glycan expression, especially in sialyl-Lex, was abrogated by treatment with benzyl-GalNAc (black lines in 4 and 8) but castanospermine did not block the reduction by the sialidase (black lines in 3 and 7).