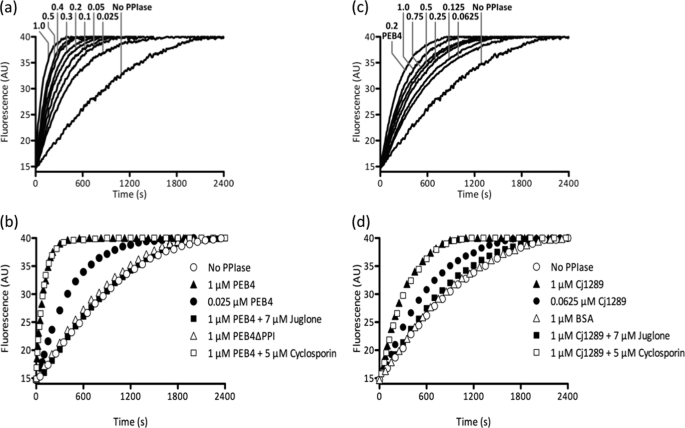
FIGURE 4.
Comparison of the PPIase activity of PEB4, PEB4ΔPPI, and Cj1289 proteins as measured by ribonuclease refolding. At time 0, refolding of RCM-RNase T1 was initiated by a 30-fold dilution to give a final concentration of 0.5 μm in the presence of 2 m NaCl. The fluorescence change was measured at 15 °C and the data shown are representative of three independent experiments. a, the refolding of RCM-RNase T1 is systematically accelerated by increasing concentrations of PEB4. The numbers on the graph are the final concentrations of PEB4 in μm. b, deletion of the PPIase domain of PEB4 (PEB4ΔPPI) abolishes the rate enhancement observed with full-length PEB4 protein. Preincubation of PEB4 with cyclosporin A, a cyclophilin inhibitor, had no effect on its ability to assist RNase T1 refolding, whereas preincubation with juglone, a parvulin inhibitor, completely eliminated the acceleration of refolding. c, as in panel a but using increasing concentrations of Cj1289 (numbers are final concentration in μm) in comparison to 0.2 μm PEB4. d, juglone inhibits the PPIase activity of Cj1289, whereas cyclosporin A is without effect. BSA is used here as an additional control to show that the rate enhancement is Cj1289 specific.