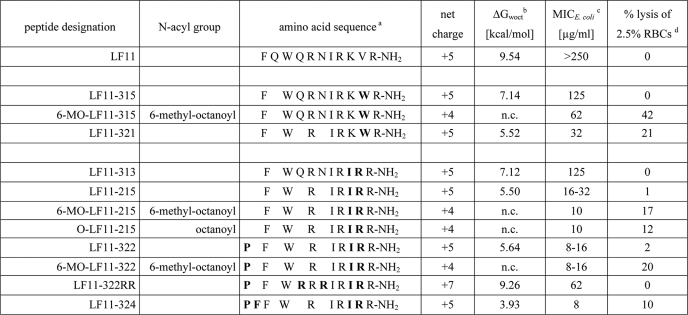
TABLE 1.
Primary structure, hydrophobicity, and biological activity of the parent peptide LF11 and its derivatives
a Substitutions and additional amino acids are given in boldface.
b Peptide hydrophobicity expressed as transfer free energy of peptides from water to n-octanol (ΔGwoct) was calculated from the whole residue hydrophobicity scale taking into account the contribution of the C-terminal amide (44). n.c. means not calculated.
c MICs against E. coli ATCC 25922 were determined as a peptide concentration resulting in less than 2% growth following an overnight incubation in Mueller Hinton medium at 37 °C in the presence of 5 × 105 colony-forming units/ml.
d Percentage of hemolysis of human RBCs was calculated following a 1-h incubation at 37 °C in PBS using 1% Triton as 100% lysis and PBS as 0% lysis; peptide concentration was 500 μg/ml.