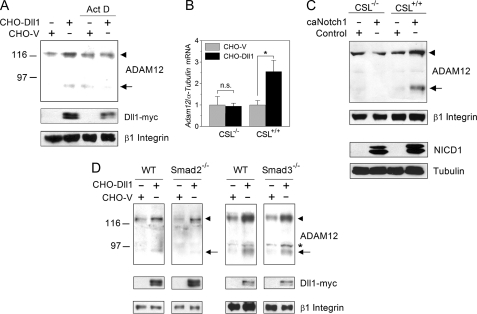
FIGURE 3.
Up-regulation of ADAM12 expression by Notch requires new transcription and is activated in a CSL-dependent, Smad2/3-independent manner. A, NIH3T3 cells were co-cultured with CHO-Dll1 or CHO-V cells for 8 h, in the absence or presence of actinomycin D (Act D), an inhibitor of transcription. The levels of ADAM12 protein and Dll1-Myc were evaluated by Western blotting after glycoprotein enrichment. The nascent form of ADAM12 is indicated by an arrowhead, the mature form of ADAM12 is shown by an arrow, and β1 Integrin indicates a gel loading control. B, CSL−/− or CSL+/+ mouse fibroblasts were co-cultured for 24 h with CHO-Dll1 or CHO-V cells. The level of Adam12 in mouse cells, normalized to α-tubulin, was evaluated by quantitative RT-PCR. The conditions of quantitative RT-PCR were optimized to ensure that the contribution of Adam12 or α-tubulin from CHO cells was negligible. The data represent the means ± S.E. from 3 independent measurements, *, p < 0.05, n.s., non-significant. C, CSL−/− or CSL+/+ fibroblasts were infected with caNotch1 retroviruses. NICD1 and the endogenous ADAM12 protein were detected by Western blotting. D, Smad2−/−, Smad3−/−, or the corresponding wild-type (WT) mouse embryonic fibroblasts were co-cultured for 24 h with CHO-Dll1 or CHO-V cells. Glycoprotein-enriched cell fractions were analyzed by Western blotting using anti-ADAM12 and anti-Myc antibodies, β1 Integrin indicates a gel loading control, and the asterisk indicates a nonspecific band. The experiments in panels A, C, and D were repeated three times with similar results.