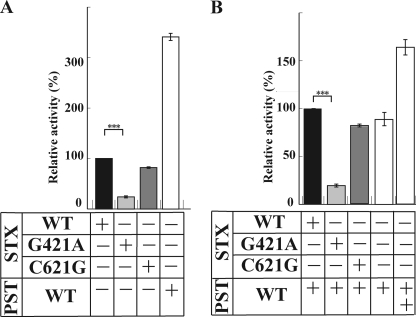
FIGURE 1.
In vitro enzyme activity of polysialyltransferases. A, in vitro enzyme activity of STX(WT), STX(G421A), STX(C621G), and PST(WT) toward NCAM-Fc. The enzyme reaction was carried out at 37 °C for 3 h in 10-μl reaction mixtures containing each enzyme, 50 mm MES buffer (pH 6.0), 10 mm MnCl2, 0.5% Triton CF-54, 10 μm CMP-[14C]Neu5Ac, and NCAM-Fc. A 5-μl aliquot was spotted on Whatman 3 MM paper and developed in ethanol, 1 m ammonium acetate (7:3, v/v), pH 7.5. After air-drying, the amount of incorporated [14C]Neu5Ac remaining at the origin was determined using a BAS 2000 imaging analyzer (Fuji Film, Tokyo, Japan). The data are expressed as the mean ± S.D. (error bars), with at least 3 replicates/experiment. The relative activity of each polysialyltransferase was determined by comparing with the activity of STX(WT), which was set to 100%. ***, p = 0.00022 (<0.001). B, in vitro enzyme activity of STX(WT), STX(G421A), and STX(C621G), in the presence of PST(WT), toward NCAM-Fc. The data are expressed as the mean ± S.D., with at least 3 replicates/experiment. The relative activity of each pair of polysialyltransferases was determined by comparing to the activity of STX(WT)/PST(WT), which was set to 100%. ***, significance at p = 0.00012 (<0.001). +, presence of enzyme. ++, presence of enzyme in a double amount. −, absence of enzyme.