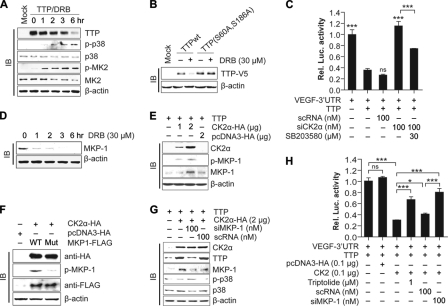
FIGURE 6.
Effects of CK2 on TTP is mediated by the MKP-1/p38 MAPK pathways. A, CK2 inhibition by DRB increases phosphorylation of p38 MAPK and MK2. Colo320 cells were transfected with pcDNA6/V5-TTP, and the cells were treated with 30 μm DRB at 24 h post-transfection. Cells were harvested at indicated times after DRB treatment, and cell lysates were analyzed for TTP, p38 MAPK, phosphorylated p38 MAPK, MK2, and phosphorylated MK2 by immunoblotting (IB). B, mutation of MK2 phosphorylation sites of TTP blocks the DRB-induced degradation of TTP. Colo320 cells were transfected with pcDNA6/V5-TTP or pcDNA6/V5-TTP(S60A and S186A) and were treated with DRB for 12 h. The expression level of the TTP protein was determined by immunoblotting with anti-V5 antibody. C, p38 MAPK inhibitor SB203580 attenuates the decrease mRNA decaying activity of TTP induced by siRNA against CK2α. Colo320 cells were cotransfected with psiCHECK2-VEGF 3′UTR, pcDNA6/V5-TTP, and siCK2α or scRNA. Cells were treated with SB203580 for 12 h, and luciferase activity was determined. Renilla luciferase activity was normalized to firefly activity. The luciferase activity obtained from cells transfected with psiCHECK2-VEGF 3′UTR was set to 1. Each bar represents the mean ± S.D. of three independent experiments (***, p < 0.001). D, inhibition of CK2α by DRB decreases the expression of MKP-1. Colo320 cells were treated with DRB, and cells were harvested at the indicated times after DRB treatment. Cell lysates were analyzed for MKP-1 by immunoblotting with anti-MKP-1 antibody. E, overexpression of CK2α increases the expression of MKP-1. Colo320 cells were cotransfected with pcDNA6/V5-TTP and pcDNA3/HA-CK2α. At 24 h post-transfection, expression of CK2α and MKP-1 was determined by immunoblotting. F, mutation of CK2 phosphorylation sites of MKP-1 blocks the CK2α-induced phosphorylation of MKP-1. Colo320 cells were cotransfected with pcDNA6/V5-TTP, pcDNA3/HA-CK2α, and pcDNA3.1/FLAG-MKP-1 or pcDNA3.1/FLAG-MKP-1 (S131A and S235A). At 24 h post-transfection, cell lysates were analyzed for CK2α, MKP-1, and phosphorylated MKP-1 by immunoblotting. G, inhibition of MKP-1 by siRNA abolishes the effects of CK2 on TTP expression and p38 MAPK phosphorylation. Colo320 cells were cotransfected with pcDNA6/V5-TTP, pcDNA3/HA-CK2α, and siRNA against MKP-1 (siMKP-1) or scRNA. At 24 h post-transfection, cell lysates were analyzed for CK2α, TTP, MKP-1, p38 MAPK, and phosphorylated p38 MAPK by immunoblotting. H, inhibition of MKP-1 by MKP-1 inhibitor triptolide or siRNA abolishes the effects of CK2α on mRNA decaying activity of TTP. Colo320 cells were cotransfected with psiCHECK2-VEGF 3′UTR, pcDNA6/V5-TTP, pcDNA3/HA-CK2α, and siMKP-1 or scRNA. Cells were treated with triptolide for 12 h, and the luciferase activity was determined. Renilla luciferase activity was normalized to firefly activity. The luciferase activity obtained from cells cotransfected with psiCHECK2-VEGF 3′UTR and pcDNA6/V5-TTP was set to 1. Each bar represents the mean ± S.D. of three independent experiments (*, p < 0.05; ***, p < 0.001). ns, not significant.