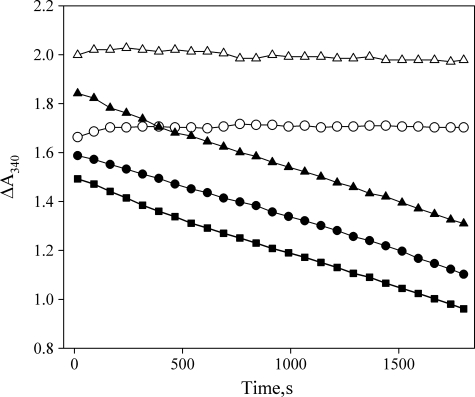
FIGURE 2.
Drosophila myosin-18 lacks actin-activated MgATPase activity. M-PDZ and M-ΔPDZ were assayed for actin-activated MgATPase activity in an NADH-coupled assay. Representative traces of data from M-PDZ (○) and M-ΔPDZ (△) motors in the absence of actin show no detectable hydrolysis of ATP. In the presence of 45 μm F-actin and 2 mm ATP, M-PDZ (●) and M-ΔPDZ (▴) show no detectable difference in the rate of change of A340 from the ATP hydrolysis rate of actin alone (■) at the same concentration. Experiments were conducted at 25 °C in a buffer containing 50 mm KCl, 10 mm MOPS (pH 7.2), 2 mm MgCl2, 0.15 mm EGTA, 2 mm ATP, 40 units/ml lactate dehydrogenase, 200 units/ml pyruvate kinase, 1 mm phosphoenolpyruvate, and 200 μm NADH. The concentration of myosin-18 fragments in each assay was 0.5 μm.