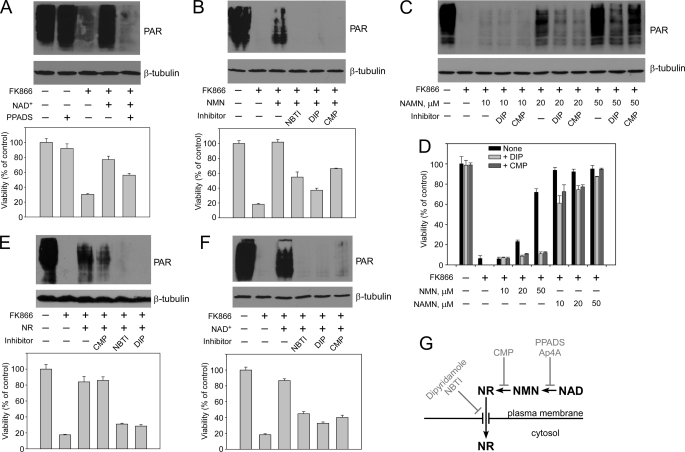
FIGURE 3.
Extracellular nucleotide precursors are degraded, and only the resultant ribosides enter the cell. Nam utilization by the 293mitoPARP cells was inhibited by FK866 addition, as indicated. Treatments with the indicated inhibitors were conducted as described under “Experimental Procedures.” The effects on mitochondrial NAD+ content (as detected by PAR formation) and cell viability are shown. A, extracellular degradation of NAD+ to NMN, which is inhibited by pyridoxyl phosphate-6-azophenyl-2′,4′-disulfonic acid (PPADS), is required to support mitochondrial NAD+ generation and cell viability. B, NMN must be dephosphorylated to NR to support mitochondrial NAD+ synthesis. Inhibitors of 5′-nucleotidase (CMP) or nucleoside transporters (dipyridamole (DIP), NBTI) were added as indicated. C, NAMN utilization requires dephosphorylation to NAR. Increasing NAMN concentrations in the medium were used to overcome the inhibition of NAD+ synthesis by DIP or CMP. D, when using NMN or NAMN as NAD+ precursors, cell viability depends on the extracellular degradation of the mononucleotides. Increasing concentrations of NMN or NAMN in the medium were used to overcome inhibition of NAD+ synthesis by DIP or CMP. The weaker inhibitory effect using NAMN as extracellular precursor may be due to the presence of NA (see text). E, extracellular NR enters cells via nucleoside transporters and supports mitochondrial NAD+ synthesis and cell viability. Nucleoside carrier inhibitors (NBTI, DIP), but not CMP, strongly reduce mitochondrial NAD+ generation when NR is used as extracellular precursor. F, extracellular NAD+ is degraded to NR to serve as precursor of intracellular NAD+. Both CMP and inhibition of nucleoside carriers strongly reduce the generation of mitochondrial NAD+ when NAD+ is added as extracellular precursor. G, schematic representation of the results. The effect of Ap4A is demonstrated in supplemental Fig. S2A.