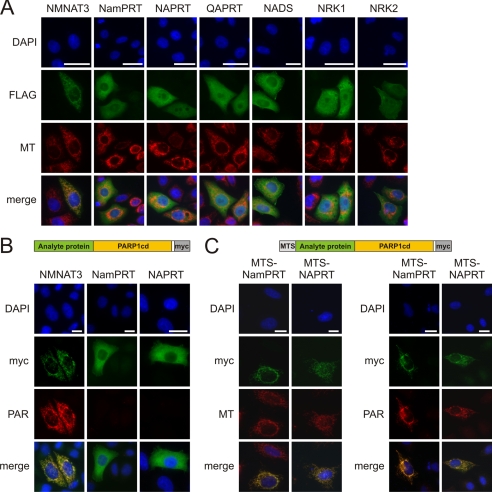
FIGURE 4.
Subcellular localization of NAD+ biosynthetic enzymes. A, all known NAD+ biosynthetic enzymes localize to the cytoplasm or the nucleus except for mitochondrial NMNAT3. HeLa S3 cells were transiently transfected with vectors encoding the indicated enzymes (C-terminally FLAG-tagged). The fluorescence micrographs show nuclei (DAPI), expressed recombinant proteins (FLAG), and mitochondria (MT). Bar, 20 μm. Mitochondrial localization of a potential variant of NAPRT was ruled out (supplemental Fig. S3A). B and C, NMNAT3, but not NamPRT or NAPRT, is a mitochondrial matrix protein. Submitochondrial protein localization was conducted following overexpression in HeLa S3 cells using PARAPLAY (33). B, analyte proteins (NMNAT3, NamPRT, and NAPRT) were overexpressed, endowed with a C-terminal PARP1cd and Myc tag (see scheme on top). If the fusion protein resides within the matrix, PAR accumulates and is detected by immunocytochemistry (as found for NMNAT3). Fusion proteins that localize to the intermembrane space or the cytosol do not support PAR accumulation (33). Bar, 20 μm. C, to verify the functionality of the PARAPLAY assay for the negative results (NamPRT and NAPRT), the corresponding constructs were additionally endowed with an established N-terminal mitochondrial targeting sequence (MTS, see scheme on top). Overexpression of these constructs resulted in mitochondrial PAR accumulation demonstrating that the NamPRT- or NAPRT-PARPcd fusion constructs are catalytically active. However, the authentic proteins (without added MTS) do not localize to the mitochondrial matrix, and therefore PAR accumulation was not observed (B).