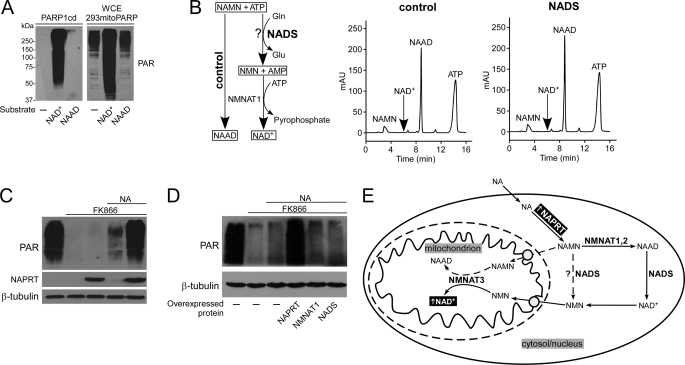
FIGURE 6.
Cytosolic nicotinic acid derivatives have to be converted to NAD+ before they can serve as mitochondrial NAD+ precursor. A, PARP1cd and mitoPARP do not synthesize PAR from NAAD. Recombinant PARP1cd or whole cell extracts (WCE) from 293mitoPARP cells were incubated with 1 mm NAD+ or NAAD. Endogenous PAR in whole cell extracts (WCE) of 293mitoPARP cells derives from PAR generated by the mitoPARP protein. As opposed to NAD+, addition of NAAD did not lead to further PAR generation. B, human NADS does not amidate NAMN. Overexpressed FLAG-tagged NADS was immunoprecipitated (supplemental Fig. S4A), and its activity to amidate NAAD to NAD+ was verified (supplemental Fig. S4B). After incubation with NAMN, ATP, and glutamine, NADS was removed by ultrafiltration, and purified NMNAT1 was added to convert NMN or NAMN to the respective dinucleotide. If NAMN had been amidated to NMN, NAD+ would be detected. The control sample did not contain NADS. C, NAPRT overexpression increases mitochondrial NAD+ formation from extracellular NA. FLAG-tagged NAPRT was transiently expressed in 293mitoPARP cells, as indicated. D, NAPRT is rate-limiting for mitochondrial NAD+ generation from extracellular NA. 293mitoPARP cells transiently expressed FLAG-tagged NAPRT, NMNAT1, or NADS, as indicated. E, NA-dependent mitochondrial NAD+ synthesis requires generation of cytosolic NAD+. Direct conversion of NAMN to NMN by NAD synthetase was excluded (dashed line). Import of NAMN into mitochondria and conversion by NMNAT3 to NAAD would not be detected (A). Therefore, NA undergoes several cytosolic conversions including NAAD amidation to NAD+ and degradation to NMN, which then enters the mitochondria. Note that use of extracellular NAR to generate mitochondrial NAD+ is similar, because this nucleoside is also first converted to NAMN by NRK (not shown).