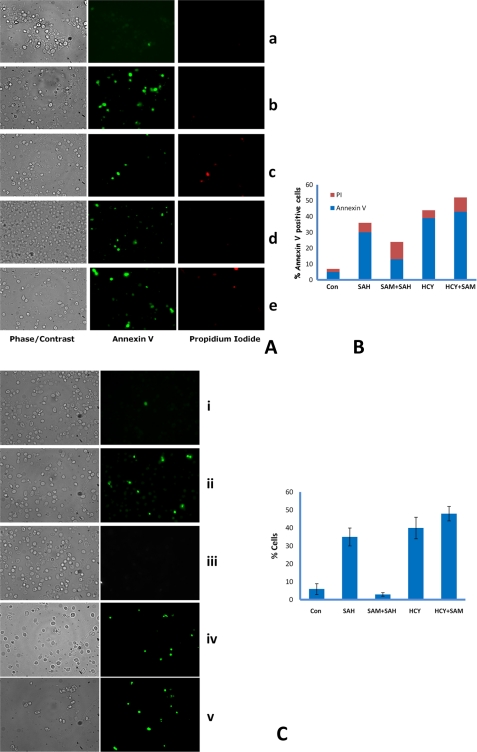
FIGURE 10.
AdoHcy- and homocysteine-treated cells exhibit the typical markers of yeast apoptosis, DNA fragmentation, and annexin V staining. A, exposition of phosphatidylserine at the membrane surface. str4Δ cells were grown in minimal media containing AdoHcy (600 μm), AdoMet (600 μm), and homocysteine (5 mm), and cells were harvested after 12 h of incubation and stained with FITC-labeled annexin V for detection of exposed phosphatidylserine and propidium iodide for detection of damaged cells. Fluorescence and differential interference contrast micrographs showing normal annexin (−), PI (−) protoplasts, apoptotic cells as annexin (+) PI (−), and dead cells as annexin (+) PI (+). Panel a, control cells; panel b, AdoHcy; panel c, AdoMet + AdoHcy; panel d, homocysteine; panel e, Hcy + AdoMet. The results reported are for three independent experiments. B, quantitation of apoptotic cells; % of annexin V-positive cells were counted from >100 cells from different field views. C, DNA strand breakage visualized by TUNEL staining in str4Δ strain. str4Δ cells were grown in minimal media containing the following: AdoHcy (SAH) (600 μm), AdoMet (SAM) (600 μm), homocysteine (5 mm); cells were harvested after 12 h of incubation and stained for DNA strand breaks with TUNEL reaction containing fluorescently tagged dUTP. Quantitation of apoptotic cells; % positive cells were counted from >120 cells. Error bars are representative of means ± S.D. (n = 3). Left panel, phase contrast microscopy; right panel, fluorescence microscopy of the same cells. Panel i, control (Con) cells; panel ii, AdoHcy; panel iii, AdoMet + AdoHcy; panel iv, homocysteine; panel v, Hcy + AdoMet.