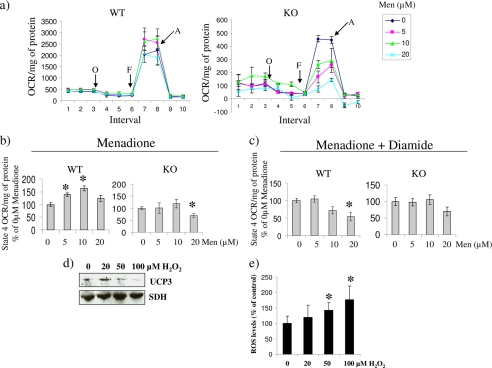
FIGURE 4.
a, in situ determination of the mitochondrial bioenergetic parameters of WT and UCP3 null (KO) primary myotubes exposed to varying amounts of menadione for 24 h. Analyses were conducted using the Seahorse extracellular flux analyzer. Following the determination of basal respiration various bioenergetic parameters were tested. State 4 respiration, maximal respiration, and extramitochondrial O2 consumption were tested using oligomycin (O; 1 μg/ml), carbonyl cyanide p-trifluoromethoxyphenylhydrazone (F; 1 μm), and antimycin A (A; 1 μm), respectively. b, state 4 respiration in WT or UCP3 null (KO) myotubes treated with menadione. State 4 respiration was induced with oligomycin. Data were expressed as a percentage of 0 μm menadione. *, p < 0.05, n = 4, one-way ANOVA with Tukey's post hoc test. The asterisk corresponds to comparison with control mean. c, impact of diamide treatment on state 4 respiration in menadione-exposed cells. Oligomycin-induced state 4 respiration was measured following a 15-min exposure to 100 μm diamide. *, p < 0.05, n = 4, one-way ANOVA with Tukey's post hoc test. The asterisk corresponds to comparison with control mean. d, ROS deglutathionylates UCP3. Primary WT myotubes were exposed to BioGEE and then incubated in H2O2 (0–100 μm). Cells were then lysed, and proteins modified by BioGEE were isolated with streptavidin beads. The presence of both UCP3 and SDH was tested by immunoblot following streptavidin pull-down. e, ROS levels in BioGEE-treated primary WT myotubes exposed to H2O2. Following treatment with BioGEE, cells were exposed to H2O2 (0–100 μm) for 15 min and then tested for ROS levels with dichlorofluorescein diacetate (20 μm, 30-min exposure). Data were normalized to protein level and expressed as a percentage of control. Absolute values were calculated by subtracting background fluorescence. *, p < 0.05, n = 6, one-way ANOVA with Tukey's post hoc test. Error bars, S.D.